Scheme 2.
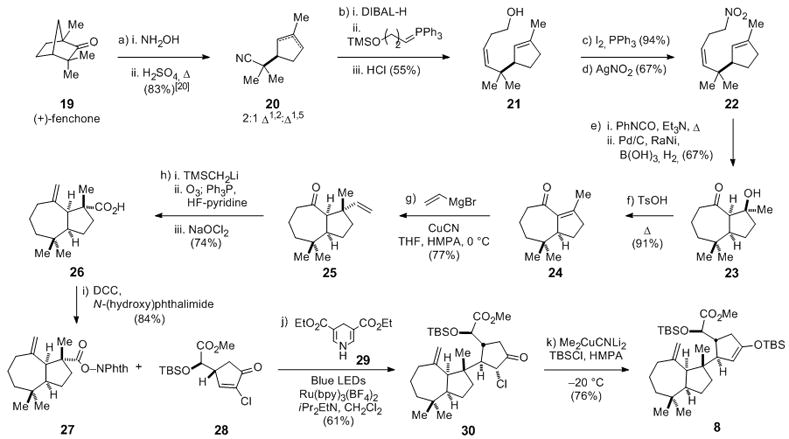
Formal total synthesis of (−)-aplyviolene: Synthesis of enoxysilane 8: a) i. 19, sodium acetate (2 equiv), NH2OH•HCl (1.75 equiv), EtOH, reflux, 36 h; ii. 4 M H2SO4, reflux, 8 h, 83% as a 2:1 mixture of Δ1,2:Δ1,5 isomers. b) i. 20, DIBAL-H (1.2 equiv), CH2Cl2, −78 °C, 1 h; ii. 3-hydroxypropyltriphenylphosphonium bromide (2 equiv), n-BuLi (4 equiv), TMSCl (2 equiv), THF, 0 °C, 20 min; + aldehyde from i., −78 °C, 1 h; 2 N H2SO4, RT, 18 h; 55%. c) 21, I2 (1.05 equiv), PPh3 (1.05 equiv), imidazole (1.1 equiv), benzene, 18 h, RT, 94%. d) alkyl iodide from c), AgNO2 (1.5 equiv), 18 h, RT, 67%. e) i. 22, PhNCO (3 equiv), Et3N (0.5 equiv), toluene, 18 h, 90 °C; ii. H2, 10% Pd/C (5 wt%), Raney-Ni (5 wt%), B(OH)3 (3 equiv), MeOH/H2O (5:1), 36 h, RT, 67% f) 23, TsOH (0.1 equiv), 5 h, 100 °C, 91%. g) 1 M vinylmagnesium bromide (5 equiv), CuCN (2.5 equiv), THF/HMPA (5:1), 10 min, 0 °C, + 24, 7 h, 0 °C, 77%. h) i. TMSCH2Li (5 equiv), pentane, −78 °C; + 25; ii. O3, CH2Cl2, −78 °C; Ph3P (1.5 equiv), HF−pyridine, 1 h, 0 °C; iii. NaClO2 (3 equiv), 2-methyl-2-butene (3 equiv), NaH2PO4 (1 equiv), acetone/H2O (30:1), RT, 1 h, 74%. i) 26, DCC (1.5 equiv), N-(hydroxy)phthalimide (1.7 equiv), DMAP (0.05 equiv), THF, 18 h, RT, 84%. j) 27, 28 (1.5 equiv), 29 (1.5 equiv), iPr2EtN (2.25 equiv), Ru(bpy)3(BF4)2 (0.01 equiv), CH2Cl2, blue LEDs, 2.5 h, RT, 61%. k) 31, Me2CuCNLi2 (2.0 equiv), TBSCl (5 equiv), Et2O/THF/HMPA (4:2:1), 1 h, −20 °C, 76%. DIBAL-H = Diisobutylaluminum hydride. TsOH = p-toulenesulfonic acid. DCC = N,N′ dicyclohexylcarbodiimide. DMAP = 4-dimethylaminopyridine. HMPA = hexamethylphosphoramide.
