Table 1.
Deoxygenation Studies for Compounds 17 and 20
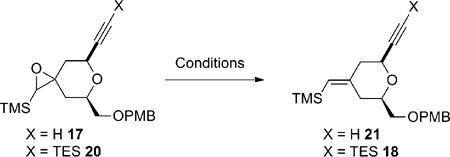 | ||||
|---|---|---|---|---|
| Entry | X | Conditions | Product | Yield |
| 1 | H | 10 mol% Rh2(OAc)4, C6H6, 80 °C | 21 | 15% |
| 2 | H | 10 mol% Rh2(OAc)4, C6H5CI, 80 °C | 21 | trace |
| 3 | H | Al/HgCI2, C6H6/i-PrOH, reflux | 21 + double bond isomer | clean reaction |
| 4 | H | WCI6, 3 equiv. n-BuLi, −78 °C → RT | baseline material | 0% |
| 5 | H | (PhO)3PCH3I, BF3•OEt2, RT | recovered 17 | 0% |
| 6 | TES | 10 mol% Rh2(OAc)4, C6H6, 80 °C | 18 | 52% |
