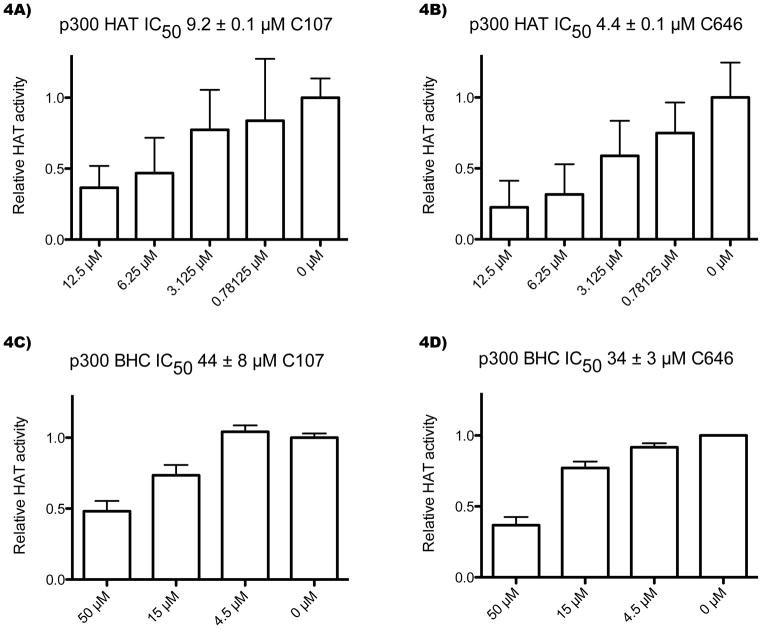
Figure 4.
C107 is an inhibitor of p300 HAT. A–B) Concentration dependence of C107 and C646 inhibition of p300 HAT domain acetyltransferase activity. C107 and C646 experiments were run in parallel. Reactions contained 10 nM p300 HAT domain, 100 μM H4 N-terminal 15-mer peptide, and C107 or C646 compound (with DMSO amounts held constant) were allowed to pre-equilibrate for 12 min, followed by a 10-min reaction at 30°C with 10 μM 14C-acetyl-CoA. The reaction mixtures were separated by Tris-Tricine gel electrophoresis quantified with a phosphorimager, and background subtracted. The averages and standard errors of quadruplicate reactions are shown, and the IC50s were calculated using a nonlinear fit in Grafit. C–D) Concentration dependence of C107 and C646 inhibition of p300 BHC (bromodomain, histone acetyltransferase domain, C/H3 domain) acetyltransferase activity. C107 and C646 experiments were run in parallel. Reactions contained 0.05 nM p300 BHC, 100 μM H4-15mer, and C107 or C646 were allowed to pre-equilibrate for 10 min, followed by a 5-min reaction at 30°C with 0.1 μM 14C-acetyl-CoA. The reactions were analyzed as above, with the C107 experiment conducted with six replicates and the C646 experiment with two replicates.