Figure 2.
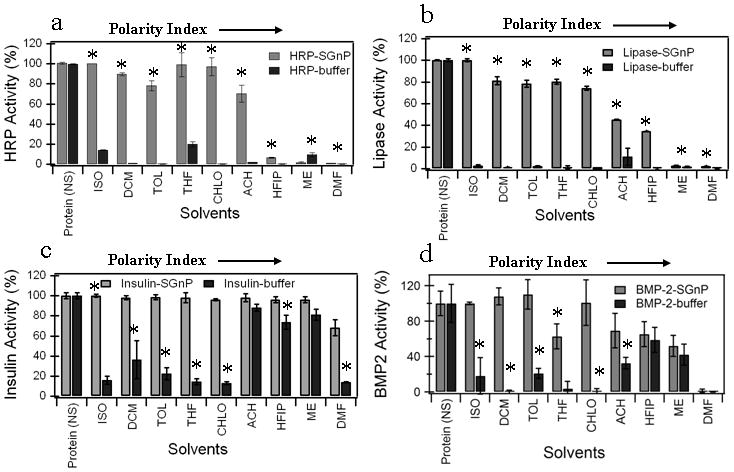
Activity retention data for four proteins; (a) HRP, (b) Lipase, (c) Insulin, and (d) BMP-2 exposed to nine solvents, either directly from buffer, or while encapsulated in SGnPs. HRP and Lipase were used as model proteins to study the enzymatic activity of the proteins whereas insulin and BMP-2 were used to study the biological activity. To compare how the SGnP system protects protein from adverse chemical environments, we incubated HRP-SGnP/lipase-SGnP/insulin-SGnP/BMP-2-SGnP in the different organic solvents. The protein in buffer (HRP-buffer/lipase-buffer/insulin-buffer/BMP-2-buffer) was used to mimic the conventional encapsulation techniques. The residual activity of the protein measured after incubated for 2 hours with different solvents in the form of protein in buffer (protein-buffer) or protein encapsulated into sugar-glass (protein-SGnP). The solvents used for this experiment have wide range of polarity index (0 to 6.4). Protein activity was measured after exposure to solvent and compared to activity values obtained before solvent exposure. Values are average ± one standard deviation of n=4 samples for each groups. Statistically significant differences (P<0.05) in protein activity between protein-buffer and protein-SGnP samples after incubation with a particular solvent are indicated by an asterisk (by t-test). [Protein (NS); protein-SGnP and protein-buffer samples without any solvent treatment, ISO; isooctane, DCM; dichloromethane, TOL; toluene, THF; tetrahydrofuran, CHLO; chloroform, ACH; acetone, HFIP;hexafluoroisopropanol, ME; methanol, DMF;dimethylformamide]
