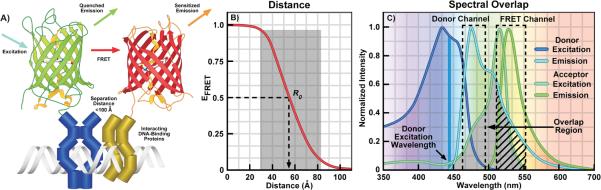
Figure 1.
A: Cartoon illustrating FRET between green and red FPs fused to interacting DNA-binding proteins. Energy transfer can only occur when the FPs are positioned close to one another by the interactions of the proteins they are fused to. The excitation of the green FP donor (cyan arrow) drives it to the excited-state, and that energy can be transferred directly to the nearby red FP acceptor by FRET. This results in quenching of the donor emission (green arrow) and sensitized emission (orange arrow) from the acceptor. B: The distance dependence for efficient FRET. The Förster equation (Box 1) was used to determine the change in FRET efficiency (EFRET) as a function of the separation distance between the FPs. The shaded region shows the range of 0.5 R0 to 1.5 R0 over which FRET can be accurately measured. C: The excitation and emission spectra for the Cerulean (donor) and Venus (acceptor) showing the spectral overlap between the donor emission and acceptor excitation (shaded region). The dashed boxes indicate the donor and FRET detection channels, the arrow indicates the direct acceptor excitation at the donor excitation wavelength, and the hatching shows donor SBT into the acceptor channel.