Figure 3.
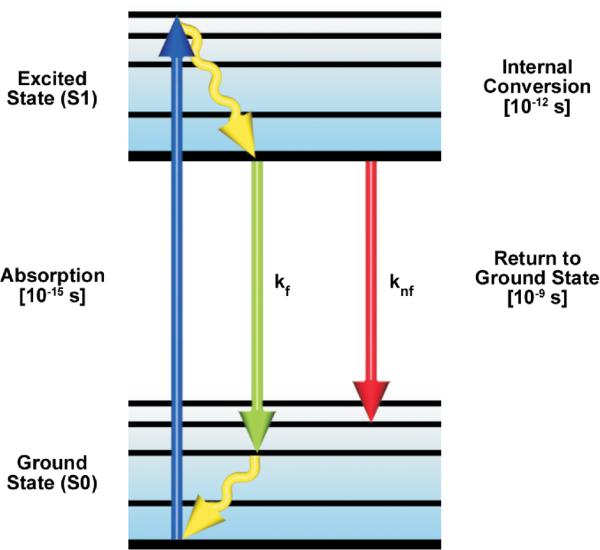
A simplified Perrin-Jablonski energy level diagram for a fluorescent molecule. The arrows represent absorption of excitation photon energy causing the transition from the lowest vibrational levels of the ground state (S0) to the excited state (S1). Thermal energy is lost by internal conversion and the transition from the excited state to the ground state is always from the lowest level of S1. The de-excitation transitions can occur by the emissive (kf) pathway or by other competing non-emissive (knf) pathways.
