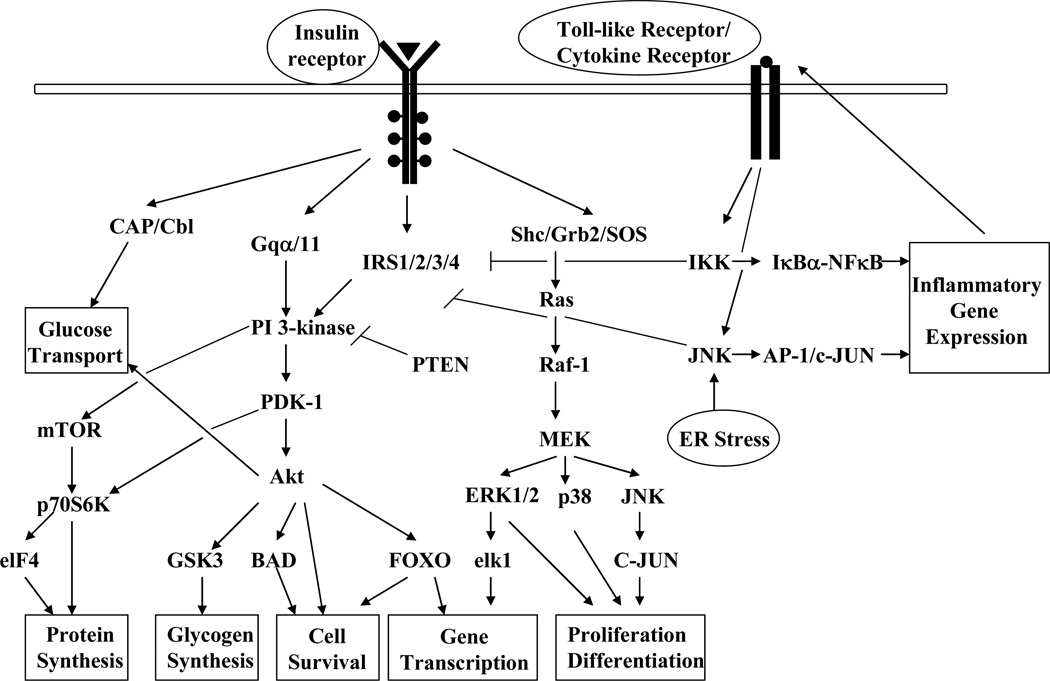
Figure 1.
Graphic illustration of the perturbation of the insulin signal transduction pathways by cellular stressors. The binding of insulin to its receptor triggers the activation of downstream signaling cascades important for the metabolic and mitogenic actions of insulin. The phosphorylation of insulin receptor substrates on select tyrosine residues leads to the activation of PI-3 kinase with subsequent increase in the activity of serine/threonine kinase Akt. Insulin also activates the Ras/MAPK signaling pathway and gene transcription. Under stressful conditions, the inflammatory pathways are activated, leading to the phosphorylation of IRS-1 at serine 307 by the kinases IKKβ and JNK1 and subsequent attenuation in insulin signal transduction. Activation of the lipid phosphatase PTEN decreases PI-3 kinase activity and, therefore, reduces Akt signaling and activation of downstream target proteins involved in multiple aspects of cell physiology.