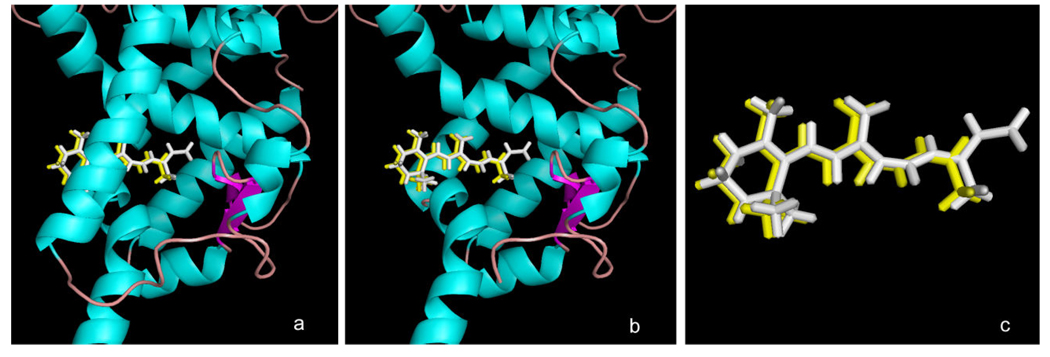
Figure 3. Docking of β-apo-13-carotenone with the RXRα tetramer.
Shown is the structure of the ligand-binding domain of the tetrameric RXRα (in green) from PDB entry 1G5Y with: (a) the bound crystallized all-trans retinoic acid (ATRA) isomer (in white) and the Surfle-Dock docked structure of β-apo-13-carotenone (yellow) when built in a conformation resembling that of the bound ATRA; (b) same as in (a) with the protein helix 3 removed to facilitate visualization of the quality of docking in the binding site; (c) as in (a) with the RXR protein removed to show details of the similarity of the crystallized ATRA ligand and the docked pose of β-apo-13-carotenone.