Figure 1.
Allelic Series of cdka;1 Mutants.
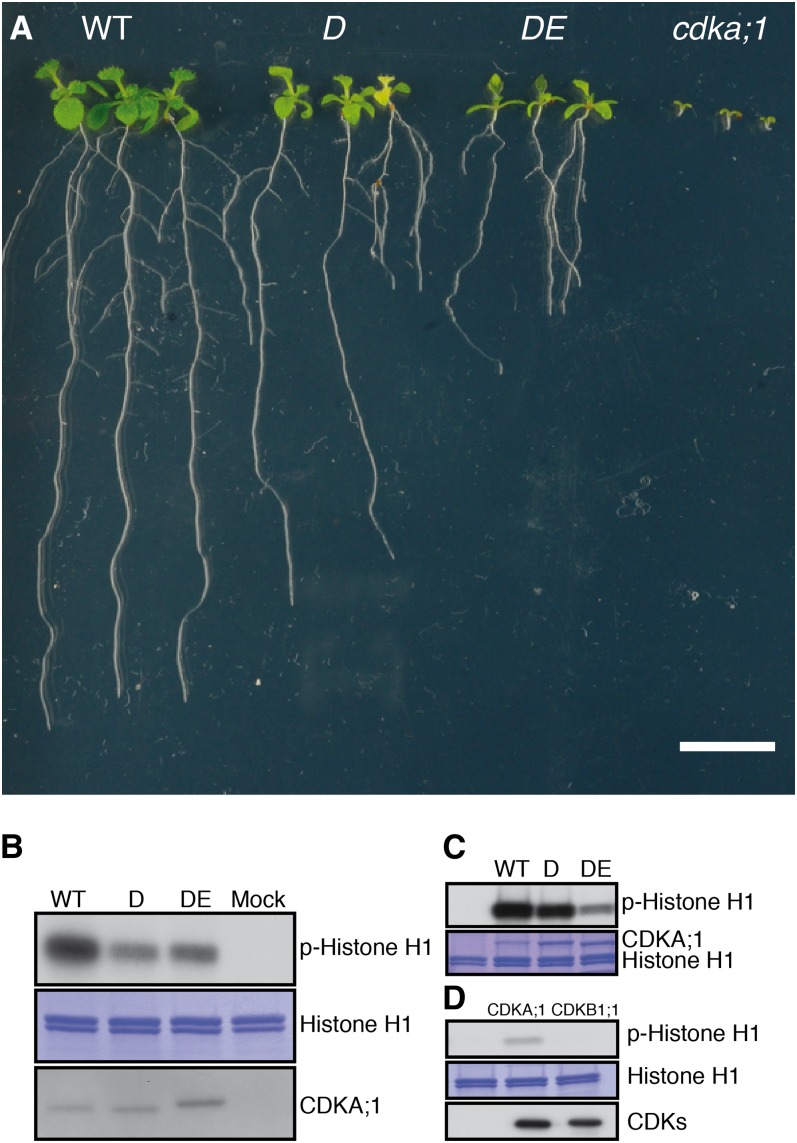
(A) Ten-day-old wild-type (WT), D, DE, and homozygous cdka;1 mutant plants (from left to right). Bar = 1 cm.
(B) p13suc1-associated protein kinase activity is strongest in wild-type extracts, while extracts from D plants have higher activity than those from DE extracts as revealed by autoradiography (top panel). Coomassie blue staining (middle panel) shows equal loading of the substrate histone H1. Equal purification levels of CDKs were quantified by protein blot with the anti-PSTAIR antibody (bottom panel). Protein extraction buffer was incubated with p13suc1 beads as a mock.
(C) In vitro kinase assays show that the hypomorphic cdka;1 mutant D has less activity than the wild type but more than the hypomorphic cdka;1 mutant DE (autoradiography top). Coomassie blue staining (bottom) shows equal loading of the substrate histone H1 and the different CDKA;1 versions.
(D) In vitro kinase assays of CDK-CYCD6;1 complexes reveal that CYCD6;1 has activity with CDKA;1 but not with CDKB1;1 against histone H1 (autoradiography top). Coomassie blue staining (middle) shows equal loading of the substrate histone H1. Quantification with Strep-Tactin HRP (bottom) reveals equal amounts of CDKA;1 and CDKB1;1 bound to CYCD6;1.