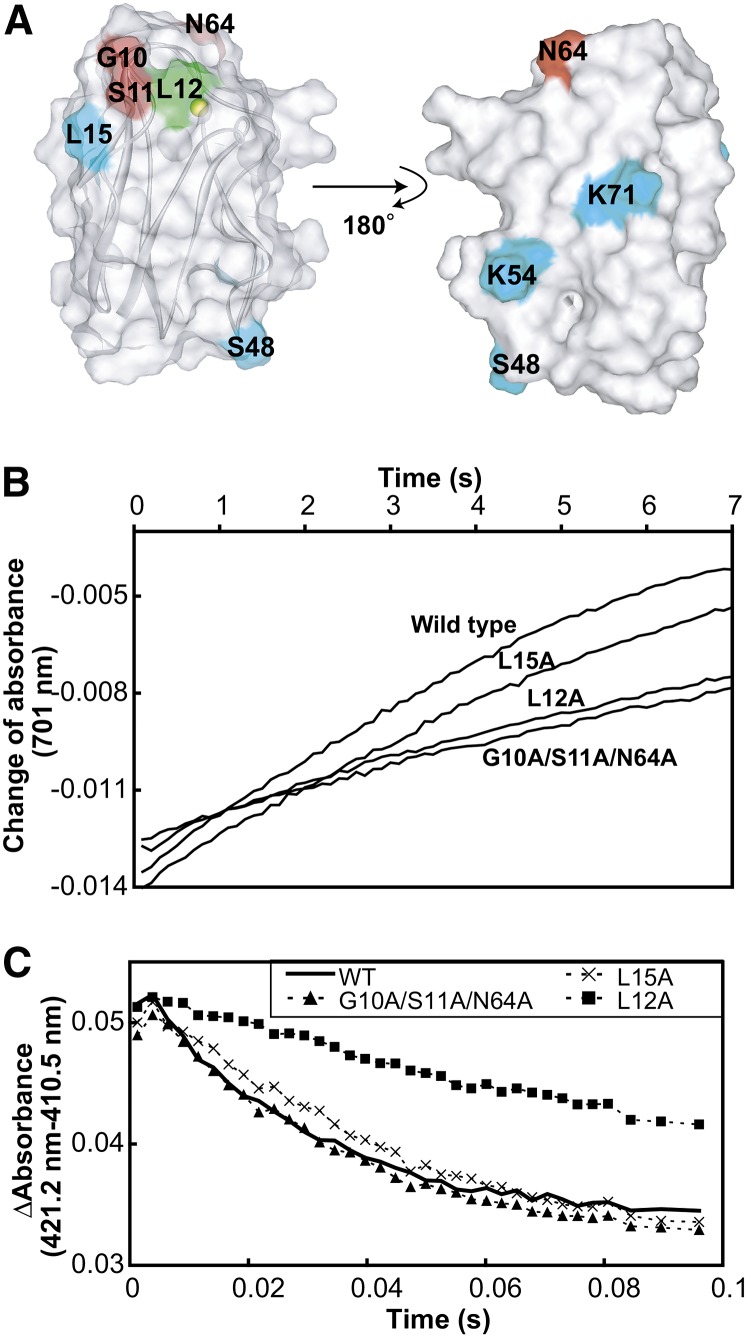
Figure 5.
Electron Transport Activities of the Wild Type and Mutant Pcs for Solubilized PSI and cyt b6f.
(A) Mapping of the mutated residues on the structure of Pc. Gly-10, Ser-11, and Asn-64, which are included in the PSI binding site but not in the cyt b6f binding site, are colored red. Leu-12, the mutation of which reportedly led to diminished electron transport activity for both PSI and cyt b6f (Sigfridsson et al., 1996; Illerhaus et al., 2000), is colored green. Leu-15, Ser-48, Lys-54, and Lys-71, which are outside the binding site, are colored cyan.
(B) Time course of the absorbance change at 701 nm of the solution containing 200 nM solubilized PSI and 40 nM wild-type and mutant Pcs after the flash. The traces were averaged from eight flash-photolysis experiments.
(C) Time courses of the absorbance change at 421.2 minus 410.5 nm, after mixing 0.15 μM reduced and solubilized cyt b6f with 1 μM oxidized wild-type and mutant Pcs. The traces were averaged from eight stopped-flow experiments.