Abstract
Objective. To evaluate the effect of an experimental gel containing Euclea natalensis extract on dentin permeability. Methods. Thirty-six dentin discs, 1-mm-thick. The discs were prepared from the coronal dentin of extracted human third molars that were divided into 3 groups (n = 10). The dentin discs in each group were treated with the groups following experimental materials: (FG): 1.23% fluoride gel, pH 4.1; (EG): Euclea natalensis extract gel, pH 4.1; (CG): control gel, pH 4.1. The gels were applied to the occlusal slide of the dentin under the following conditions: after 37% phosphoric acid and before 6% citric acid. The hydraulic conductance (HC) of each condition was determined four times using a fluid flow apparatus (Flodec). The data were analyzed using Two-way ANOVA and Tukey's test (P < 0.05). Results. The greatest mean reduction in HC was produced in group EG dentin discs (61.2%; P < 0.05). Even after acid challenge with 6% citric acid the great reduction occurred in group EG (66.0%; P < 0.05) than other groups (CG-77.1%, FG-90.8%). Conclusion. E. natalensis gel not only reduced dentin permeability, but also resisted posttreatment citric acid challenge without changing its permeability. Further research has to confirm this promising result in the clinical situation.
1. Introduction
Dentin hypersensitivity is a painful clinical condition, which affects between 4 and 5% of the adult population and is associated with dentin exposure to the oral environment [1–3]. The hydrodynamic theory predicts that exposure of dentin surfaces as a result of enamel loss and/or gingival root surface exposure resulting from attrition, abrasion, erosion, abfraction, or gingival recession can cause sensitivity [3, 4]. Thus, the concept of tubule occlusion as method of dentin desensitization is a logical correlation to the hydrodynamic theory [5]. The fact that many of the agents clinically used to desensitize dentine are effective in reducing dentin permeability tends to support the hydrodynamic theory [6]. Many agents and therapies have been proposed for the treatment of hypersensitivity, however none of them has been proven completely efficacious for such use [7], and new desensitizing agents need to be developed.
In the field of oral health researches, investigations about the contribution of natural products to the treatment of different oral diseases, such as propolis [8] and neem [9], have used experimental formulations and found that they did not cause significant side effects.
Euclea natalensis is a plant species that belongs to the family Ebenaceae common in tropical and subtropical regions of Africa, specifically on the east coast of Africa, popularly known as Mulala [10], Africans have shown the antimicrobial potential of E. natalensis roots used for oral care once daily in the morning, soon after breakfast.
A study conducted by Homer et al. [11] demonstrated that the twigs of the plant E. natalensis have sufficient antibacterial inhibitory action to interfere in virulence and growth periodontopathic bacteria, in vivo.
Several studies have evaluated the components present in the E. natalensis plant, which may be able to reduce dentin hypersensitivity. Scientific evidences have shown that E. natalensis has cytostatic properties [12], low toxicity in healthy tissues, and anti-inflammatory action [13].
Considering that the E. natalenis is used in different African provinces, and no data have yet been found on the subject in the scientific literature, it would be important to analyze the possibility of using E. natalensis for the prevention and/or control of noncarious lesions and dentin hypersensitivity. Thus, the aim of this study was to evaluate the variations in hydraulic conductance of dentin after treatment with E. natalensis gel and acidified fluorophosphate gel, in vitro. The null hypothesis tested was that the E. natalensis gel would not reduce the hydraulic conductance of dentin, in vitro.
2. Methods
2.1. Specimen Preparation and Application of Desensitizing Agents
This research was approved by the Local Research Ethics Committee (Protocol no. 178/2009). The teeth were obtained from the Human Tooth Bank of Dentistry School.
Freshly extracted human teeth are a potential source of biological pathogens [14]. Extracted human third molars were stored at 4°C in 0.1% thymol (Merck KGaA, Frankfurter Str, Darmstadt, Germany) to inhibit microbial growth and were used within 30 days after extraction.
The crowns were sectioned with a diamond saw in a precision cutting machine (Isomet 1000; Buehler, Lake Bluff, IL, USA) perpendicular to the long axis of the roots to create dentin discs from midcoronal dentin. Thirty-six dentin discs were obtained from the extracted teeth and 30 were used. The smear layer created by the diamond saw was removed with 400–600 grit SiC abrasive paper (Buehler), resulting in dentin discs approximately 0.98 ± 0.08 mm thick, as measured with a micrometer accurate to 0.01 mm. Disc surface was free of coronal enamel and with no evidence of pulp horns [7]. The specimens were immersed in 37% phosphoric acid solution for 15 s to remove the 600-grit SiC smear layer on both sides of the discs. Using 320-grit SiC abrasive paper in a rotary polisher (125 rpm) for 5 s, a standard smear layer was then produced on the occlusal surface of the discs.
To sample calculate was adopted a α error of 5% and a β error of 20%, and assumed an estimated six disks per group (SD ± 1.1).
2.2. Permeability Measurements
The rate of fluid flow through a dentin specimen was measured using the Flodec device (DeMarco Engineering, Geneva, Switzerland), which follows the movement of a tiny air bubble as it passes down a 0.6 mm diameter glass capillary located between a deionized water reservoir under 140 cm (2 psi) of water pressure and the dentin specimens [15, 16]. An infrared light source passes through the capillary and is detected by a diode, allowing the unit to follow the progress of the air bubble along the length of the capillary. Linear displacement is automatically converted to volume displacement per unit time, from which the instantaneous volumetric flow rate is calculated and logged into a spreadsheet. Flow was measured until a steady-state was reached, typically 0–5 min; then the flow was measured for at least 3 min. Since one datum was taken every second, this resulted in at least 100 readings for each condition (Figure 1). Permeability was expressed as a fluid flow rate in μL min−1. All teeth were acid-etched with 37% phosphoric acid for 30 s to ensure maximum permeability was achieved for each specimen. After etching, dentin permeability was determined. The permeability of smear layer etched dentin established a minimum permeability value for that specimen. The permeability of acid-etched dentin established the maximum permeability of each specimens [17].
Figure 1.
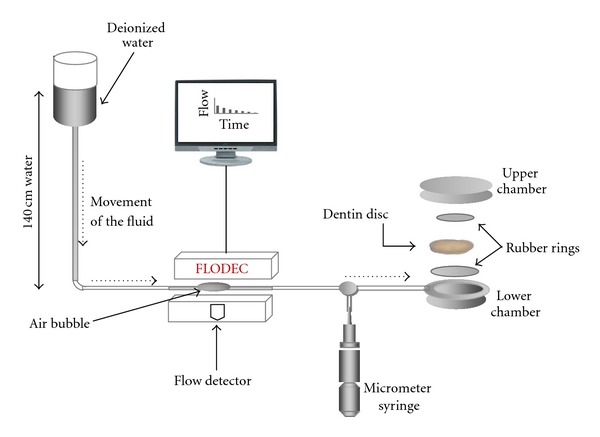
Permeability measurements adapted of Rusin et al. [15].
2.3. Application of Materials
The 30 dentin discs were randomly divided into 3 groups of 10 specimens each, corresponding to experimental group (EG): Euclea natalenis gel, group F (FG): Fluoride gel 1.23% and group C (CG): treatment with control gel. The materials used were acidulated fluorophosphate gel (1.23% acidulated fluorophosphate, pH 4.1), E. natalensis gel (10% of alcoholic extract of E. natalensis, pH 4.1), and control gel (base compounds, pH 4.1) (Table 1). The 1.23% fluoride gel was used as a control group as in other studies [7, 18] on treatment for dentin hypersensitivity.
Table 1.
Experimental design.
| Treatments | Dentinal permeability (Lp) evaluations |
|---|---|
| Smear layer (400 grit, wet sanding for 30 s) | Minimum Lp |
| 37% phosphoric acid (application for 30 s) | Maximum Lp (Lp 100%) |
| Product (application for 4 min) | Lp reduction |
| Citric acid solution 0.02 M pH 2.5 at 37-38°C (exposure for 1 min) | Lp variation |
Lp: Hydraulic conductance.
E. natalensis was kindly provided by Oral Health Department of Health Ministry of Mozambique and plant identified by Laboratory of Natural Products at Federal University of São Carlos.
The gel of E. natalensis was prepared from the dried roots of E. natalensis (under controlled parameters) and the E. natalensis extract was prepared by macerating 20.0 g of dry powder of E. natalensis root with 100 mL of 70% (w/v) ethyl alcohol for a week in a round bottom flask with occasional shaking. The flask was kept under dark to avoid effect of light on the active ingredients of the E. natalensis. The extract was then filtered through a muslin cloth for coarse residue and finally through Whatman no. 1 filter paper, measured and kept in an airtight amber colored container. Gel formulation included 10% E. natalensis extract, Carbopol, methylparaben, EDTA and sodium hydroxide solution.
All the desensitizing agents were applied for 4 min, following the manufacturer's instructions for fluorophosphate-gel and then rinsed with deionized water. After the hydraulic conductance was measured under the above conditions, the specimens were exposed to 6% citric acid pH 2.1 for 1 min and the hydraulic conductance was measured again. The aim of this treatment was to evaluate the resistance of the possible occlusive effect of the studied materials to an acidic environment, similar to that found in the mouth. The hydraulic conductance of each condition was determined four times in succession and then the mean value of the maximum Lp was calculated.
2.4. Statistical Analysis
The hydraulic conductivity measurement was performed immediately after treatment application, calculated in mL/min, and expressed as a percentage of maximum permeability obtained after etching. For each specimen, the permeability was measured four times: (1) afterthe smear layer creation, (2) after acid etching of dentin, (3) after applying the gel, and (4) after conditioning with citric acid.
Statistica 9.1 software (Stat Soft, USA) was used. The data were tested by two-way Analysis of Variance (ANOVA), at a 5% level of significance, applied to the reductions in hydraulic conductance, in order to detect differences between the conditions studied. Significant differences were found between materials, conditions, and interaction materials X conditions. Differences were identified by individual comparisons using Tukey's test at a 5% level of significance.
3. Results
The effectiveness of the three gels in reducing dentin permeability was analyzed by performing ANOVA for two criteria (P < 0.05) (Table 2). Significant differences in the reduction of dentin permeability were found among the tested gels and conditions (P < 0.000).
Table 2.
The gels were compared to the reductions in dentinal permeability between the conditions by two-way ANOVA.
| df | MS | df | SS | F-ratio | P | |
|---|---|---|---|---|---|---|
| effect | effect | error | error | |||
| Gels | 2 | 187.9275 | 36 | 3.0799 | 49.1870 | 0.0000* |
| Conditions | 3 | 63.6139 | 116 | 10.0409 | 16.6481 | 0.00000* |
| Interaction |
6 | 18.5951 | 108 | 4.9125 | 4.8664 | 0.00011* |
df: Degrees of freedom; MS: Mean square; SS: Standard error.
*Significant differences.
The results showed that although the procedures caused some reduction in the hydraulic conductance of dentin etched with phosphoric acid, the groups presented significant reduction when compared with each other (P < 0.05). Tukey's analysis identified significant differences between interactions (conditions and gels) (P < 0.05).
Table 3 shows the mean hydraulic conductance (±SD) of the groups. The desensitizing effects of gels were observed for the groups (CG = 1.63 ± 1.18; FG = 6.29 ± 2.94; EG = 0.92 ± 0.69), which behaved differently and differed significantly from each other.
Table 3.
Means (%) of hydraulic conductance (±SD) for each gels type as an after acid challenge as well as minimum and maximum (100%) hydraulic conductance and Tukey's test compared.
| Hydraulic conductance | |||
|---|---|---|---|
| Groups | Conditions | values % | μL min−1 140 cm H2O−1 |
| Control group (base gel) | Smear layer-covered dentin | 100.0 | 2.47 ± 1.32A |
| 37% PA etched dentin | 9.2 | 0.20 ± 0.22B | |
| Treatment with control gel | 73.8 | 1.63 ± 1.18Aa | |
| Posttreatment 6% CA etched | 1.90 | 1.90 ± 0.51A | |
|
| |||
| Fluoride gel group | Smear layer-covered dentin | 100.0 | 7.04 ± 1.53C |
| 37% PA etched dentin | 10.5 | 0.60 ± 0.83B | |
| Treatment with F gel | 89.3 | 6.29 ± 1.94Cb | |
| Posttreatment 6% CA etched | 90.8 | 6.32 ± 1.77C | |
|
| |||
| Euclea natalensis gel group | Smear layer-covered dentin | 100.0 | 2.53 ± 1.15A |
| 37% PA etched dentin | 20.1 | 0.36 ± 0.50B | |
| Treatment with EN gel | 61.2 | 0.92 ± 0.69Bc | |
| Posttreatment 6% CA etched | 66.0 | 1.01 ± 0.70B | |
*Different lower case letters in the same column indicate statistical significance between the gels. Different upper case letters in the same column indicate statistical significance among the different conditions (P < 0.05).
There was significant difference in dentin permeability between smear layer and tested gels (P < 0.05). Table 3 shows that the effect of gels after citric acid challenge revealed differences among the groups (CG = 77.1%; FG = 90.8%; EG = 66.0%) (P < 0.05). The EG group provided a reduction in permeability even after the acid challenge.
4. Discussion
There are diverse indications for the use of plant extracts, with very common multiplant regimens used in many traditional South African medicinal treatments. Several African tribes use the common traditional chewing stick scientifically known as E. natalensis [19]. The present plant extracts showed moderate cytotoxicity on the Vero cell line [20].
Natural products, such as propolis gel [8], have been developed to reduce dentin hypersensitivity. Nevertheless, therapeutic intervention with desensitizing agents may provide only partial pain relief and recurrence is common [21, 22].
In the present study, gel was used as a vehicle to deliver E. natalensis to the dental structure, in order to prevent further dentin permeability. Thus the null hypothesis was rejected.
The FG and EG presented significant difference from CG (P < 0.05) in the reduction of dentin permeability. Treatment in the control group was based on gel, prepared with same compounds as used for the FG and EG, but without the therapeutic agents. The physical benefit provided by the vehicle was similar in all the groups, removing the intergroup error analysis. The fluoride gel was worse for preventing dentin permeability (90.8%), followed by the control gel (77.1%), and the E. natalensis was the most effective (66.0%). Among the conditions, there was no significant difference as regards smear layer, gels, and acid challenge, except for 37% phosphoric acid (P < 0.05).
E. natalensis presented the most effective action to reduce dentin permeability. This effect can be attributed to the flavonoids, taninus, and naphthoquinones present in the plant roots [10]. These E. natalensis compounds may be responsible for the dentin tubule obliteration due to the formation of a protective layer on the teeth [23].
A survey of the literature has shown that natural products such as flavonoids [24], terpenoids, naphthoquinones acetylenes, and tropolones have been identified. Naphthoquinones are widely distributed in plants [25] and many are found to exhibit an interesting range of pharmacological properties including antibacterial [26], antiviral [27], trypanocidal [28], anticancer [29], and antifungal activity [30, 31].
Naphthoquinone 7-methyljuglone (5-hydroxy-7-methyl-1,4-aphthoquinone) has previously been isolated and identified as an active component of root extracts of E. natalensis [32], which could be considered the most active compound [10]. This compound may act as a potent antioxidant which significantly interferes with the apoptotic event [33].
The use of citric acid after the experimental gels is made in order to simulate the gel strength of the acid attacks normally undergoes a tooth when present in the oral cavity during ingestion of food or beverages. The results showed no significant difference between the values obtained after the application of gels and after application of citric acid, which proves that all the gels tested offered some resistance to acid challenge. The protection of the E. natalensis gel may be due to presence of tannins and flavonoids. Tannins supposedly have an astringent effect on the mucous membrane [34, 35], and the interaction of tannins with salivary proteins causes the aggregation and precipitation of protein-tannin complexes [35] that form a layer over enamel, thus providing protection against dental caries [36], and this property could also help to prevent dental wear [23, 37]. The organic degradation of dentin might also be affected by other host-derived enzymes, such as matrix metalloproteinases (MMPs), which are present in saliva and dental hard tissues [38]. Some flavonoids, as green tea polyphenols, could inhibit activity against MMPs [37, 38].
The limitations imposed by the method should be considered, thus they depended on an initial clustering of the specimens, which were standardized for all other comparisons, starting with the smear layer, phosphoric acid gel and finally citric acid application [39]. In vitro method is an initial phase to test a new product to a problem, thus many characteristics of in vivo methods, as the presence of saliva, is difficult to be reproduced in the laboratory. Thus others studies, such in situ, should be developed to get results more compatible to clinical environment.
The minimum permeability of the specimens is determined by the presence of the smear layer occluding the dentin tubules, allowing minimal movement of fluids through the dentin. One study reported that the presence of smear layer on the surface of dentin is responsible for most of the total resistance to the movement of fluids within the dentin. The maximum permeability is determined by removing the smear layer by demineralization, which causes a significant increase in the filtration of fluid through the dentin [40].
E. natalensis causes light yellow-spots on the teeth and in the mouth. This transitory staining [13] is caused by flavonoids, however, it disappear within hours [20]. The colors of flavonoids are directly related to the environment and show an intense yellow at an alkaline pH and disappear in the presence of acid [41].
E. natalensis gel exhibited good activity after acid challenge to reduce dentin permeability, while maintaining similar protection when applying the gel. Further studies in vitro and in situ are needed to evaluate the properties of the E. natalensis extract effect on dentin permeability. This present research has pioneered the use of gel containing E. natalensis as a treatment for dentin hypersensitivity.
These findings are very important, especially for public health in Africa, where the E. natalensis roots are easily obtainable and used as tools for performing oral hygiene. There are additional benefits, as the E. natalensis root extract is a natural remedy for diarrhea, gonorrhea, bronchitis, pleurisy, asthma, chronic urinary tract infections, and for relieving headache and toothache [42], and also contributes to oral health.
5. Conclusion
Within the limitations of this study, the experimental E. natalensis gel formulation can interfere in the reduction/prevention of dentin permeability, even when submitted to acids challenges, and therefore the null hypothesis tested was rejected. The E. natalensis gel has provided a mechanical blocking of the experimental dentin specimen. Plant extracts used traditionally for the prevention and treatment of oral problems could be used to reduce dentin hypersensitivity. Further research has to confirm this promising result in the clinical situation.
Funding
Financial support for this study was provided by an operating Grant from the FAPESP (Grant nos. 2009/16079-0 and 2010/09951-0).
Conflict of Interests
The authors do not have any conflict of interests.
References
- 1.Pashley DH, Tay FR, Haywood VB, Collins MA, Drisko CL. Consensus-based recommendations for the diagnosis and management of dentin hypersensitivity. Compendium of Continuing Education in Dentistry. 2008;29(8):1–35. [Google Scholar]
- 2.Markowitz K, Pashley DH. Personal reflections on a sensitive subject. Journal of Dental Research. 2007;86(4):292–295. doi: 10.1177/154405910708600401. [DOI] [PubMed] [Google Scholar]
- 3.Addy M. Dentine hypersensitivity: definition, prevalence, distribution and etiology. In: Addy M, Embery G, Edgar WM, Orchardson R, editors. Tooth Wear and Sensitivity. New York, NY, USA: Martin Dunitz; 2000. pp. 239–248. [Google Scholar]
- 4.Brännstrom M, Lindén LA, Johnson G. Movement of dentinal and pulpal fluid caused by clinical procedures. Journal of Dental Research. 1968;47(5):679–682. doi: 10.1177/00220345680470050201. [DOI] [PubMed] [Google Scholar]
- 5.Pashley DH. Dentin permeability, dentin sensitivity, and treatment through tubule occlusion. Journal of Endodontics. 1986;12(10):465–474. doi: 10.1016/S0099-2399(86)80201-1. [DOI] [PubMed] [Google Scholar]
- 6.Gillam DG, Orchardson R. Advances in the treatment of root dentine sensitivity: mechanisms and treatment principles. Endodontic Topics. 2006;13(1):13–33. [Google Scholar]
- 7.Santiago SL, Pereira JC, Martineli ACBF. Effect of commercially available and experimental potassium oxalate-based dentin desensitizing agents in dentin permeability: influence of time and filtration system. Brazilian Dental Journal. 2006;17(4):300–305. doi: 10.1590/s0103-64402006000400007. [DOI] [PubMed] [Google Scholar]
- 8.Sales-Peres SH, Carvalho FN, Marsicano JA, et al. Effect of propolis gel on the in vitro reduction of dentin permeability. Journal of Applied Oral Science. 2011;19(4):318–323. doi: 10.1590/S1678-77572011005000004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sales-Peres AC, Marsicano JA, Garcia RP, et al. Effect of natural gel product on bovine dentin erosion in vitro. Journal of Applied Oral Science. 2012;20(6) doi: 10.1590/1679-775720130242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McGaw LJ, Lall N, Hlokwe TM, Michel AL, Meyer JJM, Eloff JN. Purified compounds and extracts from Euclea species with antimycobacterial activity against Mycobacterium bovis and fast-growing mycobacteria. Biological and Pharmaceutical Bulletin. 2008;31(7):1429–1433. doi: 10.1248/bpb.31.1429. [DOI] [PubMed] [Google Scholar]
- 11.Homer KA, Manji F, Beighton D. Inhibition of protease activities of periodontopathic bacteria by extracts of plants used in Kenya as chewing sticks (mswaki) Archives of Oral Biology. 1990;35(6):421–424. doi: 10.1016/0003-9969(90)90203-m. [DOI] [PubMed] [Google Scholar]
- 12.Evans WC. Pharmocognosy. Philadelphia, Pa, USA: Saunders; 2002. [Google Scholar]
- 13.Filipe M, Gomes ET, Serrano R, Silva O. Characterization pharmacognostic of Euclea natalensis root. Proceedings of the Workshop herbal and medicinal plants in the Tropics (IICT /CCCM '08); 2008; p. 11 pages. [Google Scholar]
- 14.Preston KP, Higham SM, Smith PW. The efficacy of techniques for the disinfection of artificial sub-surface dentinal caries lesions and their effect on demineralization and remineralization in vitro. Journal of Dentistry. 2007;35(6):490–495. doi: 10.1016/j.jdent.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 15.Rusin RP, Agee K, Suchko M, Pashley DH. Effect of a new desensitizing material on human dentin permeability. Dental Materials. 2010;26(6):600–607. doi: 10.1016/j.dental.2010.02.010. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Y, Agee K, Pashley DH, Pashley EL. The effects of Pain-Free desensitizer on dentine permeability and tubule occlusion over time, in vitro. Journal of Clinical Periodontology. 1998;25(11):884–891. doi: 10.1111/j.1600-051x.1998.tb02386.x. [DOI] [PubMed] [Google Scholar]
- 17.Bouillaguet S, Duroux B, Ciucchi B, Sano H. Ability of adhesive systems to seal dentin surfaces: an in vitro study. Journal of Adhesive Dentistry. 2000;2(3):201–208. [PubMed] [Google Scholar]
- 18.Aranha ACC, Freire Pimenta LA, Marchi GM. Clinical evaluation of desensitizing treatments for cervical dentin hypersensitivity. Brazilian Oral Research. 2009;23(3):333–339. doi: 10.1590/s1806-83242009000300018. [DOI] [PubMed] [Google Scholar]
- 19.Lall N, Meyer JJM. Antibacterial activity of water and acetone extracts of the roots of Euclea natalensis. Journal of Ethnopharmacology. 2000;72(1-2):313–316. doi: 10.1016/s0378-8741(00)00231-2. [DOI] [PubMed] [Google Scholar]
- 20.Stander I, Van Wyk CW. Toothbrushing with the root of Euclea natalensis. Journal de Biologie Buccale. 1991;19(2):167–172. [PubMed] [Google Scholar]
- 21.Dababneh RH, Khouri AT, Addy M. Dentine hypersensitivity-an enigma? A review of terminology, epidemiology, mechanisms, aetiology and management. British Dental Journal. 1999;187(11):606–611. doi: 10.1038/sj.bdj.4800345. [DOI] [PubMed] [Google Scholar]
- 22.Walters PA. Dentinal hypersensitivity: a review. Journal of Contemporary Dental Practice. 2005;6(2):107–117. [PubMed] [Google Scholar]
- 23.Sales-Peres SHC, Pessan JP, Buzalaf MAR. Effect of an iron mouthrinse on enamel and dentine erosion subjected or not to abrasion: an in situ/ex vivo study. Archives of Oral Biology. 2007;52(2):128–132. doi: 10.1016/j.archoralbio.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 24.Moon YJ, Wang X, Morris ME. Dietary flavonoids: effects on xenobiotic and carcinogen metabolism. Toxicology in Vitro. 2006;20(2):187–210. doi: 10.1016/j.tiv.2005.06.048. [DOI] [PubMed] [Google Scholar]
- 25.Thomson R. Naturally Occurring Quinones. London, UK: Academic Press; 1971. [Google Scholar]
- 26.Osman SAA, Abdalla AA, Alaib MO. Synthesis of sulfanilamido-naphthoquinones as potential antituberculous agents. Journal of Pharmaceutical Sciences. 1983;72(1):68–71. doi: 10.1002/jps.2600720116. [DOI] [PubMed] [Google Scholar]
- 27.Brinkworth RI, Fairlie DP. Hydroxyquinones are competitive non-peptide inhibitors of HIV-1 proteinase. Biochimica et Biophysica Acta. 1995;1253(1):5–8. doi: 10.1016/0167-4838(95)00183-u. [DOI] [PubMed] [Google Scholar]
- 28.Salmon-Chemin L, Buisine E, Yardley V, et al. 2- and 3-substituted 1,4-naphthoquinone derivatives as subversive substrates of trypanothione reductase and lipoamide dehydrogenase from Trypanosoma cruzi: synthesis and correlation between redox cycling activities and in vitro cytotoxicity. Journal of Medicinal Chemistry. 2001;44(4):548–565. doi: 10.1021/jm001079l. [DOI] [PubMed] [Google Scholar]
- 29.Hazra B, Sur P, Roy DK. Biological activity of diospyrin towards Ehrlich ascites carcinoma in Swiss A mice. Planta Medica. 1984;50(4):295–297. doi: 10.1055/s-2007-969713. [DOI] [PubMed] [Google Scholar]
- 30.Khan MR, Mutasa SL, Ndaalio G, Wevers H. Antibiotic action and constituents of the root bark of Euclea natalensis A. Dc. Pakistan Journal of Science and Industrial Research. 1978;21:197–199. [Google Scholar]
- 31.Drewes SE, Khan F, Van Vuuren SF, Viljoen AM. Simple 1,4-benzoquinones with antibacterial activity from stems and leaves of Gunnera perpensa. Phytochemistry. 2005;66(15):1812–1816. doi: 10.1016/j.phytochem.2005.05.024. [DOI] [PubMed] [Google Scholar]
- 32.Mahapatra A, Mativandlela SPN, Binneman B, et al. Activity of 7-methyljuglone derivatives against Mycobacterium tuberculosis and as subversive substrates for mycothiol disulfide reductase. Bioorganic and Medicinal Chemistry. 2007;15(24):7638–7646. doi: 10.1016/j.bmc.2007.08.064. [DOI] [PubMed] [Google Scholar]
- 33.Li YB, Lin ZQ, Zhang ZJ, et al. Protective, antioxidative and antiapoptotic effects of 2-methoxy-6-acetyl- 7-methyljuglone from polygonum cuspidatum in PC12 Cells. Planta Medica. 2011;77(4):354–361. doi: 10.1055/s-0030-1250385. [DOI] [PubMed] [Google Scholar]
- 34.McRae JM, Kennedy JA. Wine and grape tannin interactions with salivary proteins and their impact on astringency: a review of current research. Molecules. 2011;16(3):2348–2364. doi: 10.3390/molecules16032348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bajec MR, Pickering GJ. Astringency: mechanisms and perception. Critical Reviews in Food Science and Nutrition. 2008;48(9):858–875. doi: 10.1080/10408390701724223. [DOI] [PubMed] [Google Scholar]
- 36.Prashant GM, Chandu GN, Murulikrishna KS, Shafiulla MD. The effect of mango and neem extract on four organisms causing dental caries: streptococcus mutans, Streptococcus salivavius, Streptococcus mitis, and Streptococcus sanguis: an in vitro study. Indian Journal of Dental Research. 2007;18(4):148–151. doi: 10.4103/0970-9290.35822. [DOI] [PubMed] [Google Scholar]
- 37.Barbosa CS, Kato MT, Buzalaf MA. Effect of supplementation of soft drinks with green tea extract on their erosive potential against dentine. Australian Dental Journal. 2011;56(3):317–321. doi: 10.1111/j.1834-7819.2011.01338.x. [DOI] [PubMed] [Google Scholar]
- 38.Magalhães AC, Wiegand A, Rios D, Hannas A, Attin T, Buzalaf MAR. Chlorhexidine and green tea extract reduce dentin erosion and abrasion in situ. Journal of Dentistry. 2009;37(12):994–998. doi: 10.1016/j.jdent.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 39.Pashley DH, Galloway SE. The effects of oxalate treatment on the smear layer of ground surfaces of human dentine. Archives of Oral Biology. 1985;30(10):731–737. doi: 10.1016/0003-9969(85)90185-2. [DOI] [PubMed] [Google Scholar]
- 40.Pashley DH, Livingston MJ, Greenhill JD. Regional resistances to fluid flow in human dentine in vitro. Archives of Oral Biology. 1978;23(9):807–810. doi: 10.1016/0003-9969(78)90159-0. [DOI] [PubMed] [Google Scholar]
- 41.Volp ACP, Renhe IRT, Barra K, Stringueta PC. Flavonoids anthocyanins: characteristics and properties in nutrition and health. Revista Brasileira de Nutrição Clínica. 2008;23(2):141–149. [Google Scholar]
- 42.Palgrave KC, Drummond RB. Trees of Southern Africa. Devon, UK: C. Struik; 1977. [Google Scholar]


