Abstract
Purpose.
Cornea confocal microscopy is emerging as a clinical tool to evaluate the development and progression of diabetic neuropathy. The purpose of these studies was to characterize the early changes in corneal sensitivity and innervation in a rat model of type 1 diabetes in relation to standard peripheral neuropathy endpoints and to assess the effect of Ilepatril, a vasopeptidase inhibitor which blocks angiotensin converting enzyme and neutral endopeptidase, on these endpoints.
Methods.
Streptozotocin-diabetic rats 8 weeks duration were treated with or without Ilepatril for the last 6 weeks of the experimental period. Afterwards, standard diabetic neuropathy endpoints, subbasal corneal nerves and innervation of the epithelium, corneal sensitivity using a Cochet-Bonnet esthesiometer, and vascular reactivity of the posterior ciliary artery were examined.
Results.
Diabetes caused a decrease in nerve conduction velocity, thermal hypoalgesia, and a reduction in intraepidermal nerve fiber profiles. In the cornea there was a decrease in corneal nerve fibers innervating the epithelium and corneal sensitivity, but subbasal corneal nerve fibers was not changed. Vascular relaxation in response to acetylcholine was decreased in the posterior ciliary artery. These defects were partially to completely prevented by Ilepatril treatment.
Conclusions.
These studies suggest that in type 1 diabetic rats decreased innervation of the cornea epithelium occurs early in diabetes and prior to a detectable decrease in subbasal corneal nerves and that these and other diabetic neuropathy-related defects can be partially to completely prevented by a vasopeptidase inhibitor.
Our studies demonstrated that decreased innervation of the corneal epithelium occurs early in diabetic rats and is accompanied by a decrease in corneal sensitivity and precedes changes in nerve morphometry of the subbasal layer of the cornea.
Introduction
Diabetic neuropathy is a common complication of diabetes with no known treatment.1 Translation of effective treatments of diabetic animal models to humans has failed in clinical trials.2 This is due in part to endpoints in animal studies that were insensitive when applied in human studies.2 To address this issue corneal confocal microscopy has emerged as a tool to measure small nerve fiber damage as a surrogate marker for the early detection of diabetic neuropathy.3–8 Previously we compared the effect of diabetes on standard nerve functional endpoints in a rat model of type 2 diabetes to changes in subbasal corneal innervation and corneal sensitivity.9 We found that after 20 weeks of a high fat diet and 12 weeks of hyperglycemia there was a decrease in corneal nerve fiber length and a decrease in corneal sensitivity as well as deficits in nerve conduction velocity, thermal hypoalgesia, and decrease in intraepidermal nerve fiber density in the hindpaw. In the present study we sought to determine the early effect of diabetes on subbasal corneal nerves, corneal nerves innervating the epithelium, and corneal sensitivity. We predict that decreased innervation of the epithelium occurs early in diabetes, as does loss of intraepidermal nerve fibers in the skin, and prior to a decrease in subbasal corneal nerve fibers. In these studies we also sought to determine if treating diabetic rats with Ilepatril prevents loss of nerve fibers in the cornea and preserves corneal sensitivity. We have demonstrated that Ilepatril, a vasopeptidase inhibitor that inhibits angiotensin converting enzyme and neutral endopeptidase, is an effective treatment for diabetic neuropathy as determined by evaluation of nerve conduction velocity, thermal nociception, and intraepidermal nerve fiber density of the skin from the hindpaw.2,10–12
Methods
Unless stated otherwise all chemicals used in these studies were obtained from Sigma Chemical Co. (St. Louis, MO).
Animals
Male Sprague-Dawley (Harlan Sprague Dawley, Indianapolis, IN) rats 10 to 11 weeks of age were housed in a certified animal care facility and food (Harlan Teklad, #7001, Madison, WI) and water were provided ad libitum. All institutional guidelines for use of animals were followed. The studies performed adhered to the ARVO position for the use of animals in ophthalmic and vision research. At 12 weeks of age the rats were separated into two groups. One of these groups was treated with streptozotocin (55 mg/kg in 0.9% NaCl intraperitoneally [IP]) to induce type 1 diabetes. The other group was treated with vehicle and served as the control. Diabetes was verified 48 hours later by evaluating blood glucose levels with the use of glucose-dehydrogenase–based reagent strips (Aviva Accu-Chek; Roche, Mannheim, Germany). Rats having blood glucose level of 300 mg/dL or greater were considered to be diabetic. Once diabetes was confirmed, all diabetic rats were treated daily with 2 units of Lantus insulin glargine (Sanofi-Aventis, Bridgewater, NJ) per day to maintain body weight. Insulin treatment did not prevent hyperglycemia.13 After 2 weeks diabetic rats were divided into two groups. One group was maintained on the normal diet and the other group was treated with Ilepatril (500 mg/kg in the diet) for 6 weeks.10–12 Thus, the total duration of diabetes was 8 weeks.
Thermal Nociceptive Response, Tear Secretion, and Corneal Sensitivity
Thermal nociceptive response in the hindpaw was measured using the Hargreaves method as previously described.12,14 Data were reported in seconds. Tear secretion was determined using phenol red–impregnated cotton threads (Zone-Quick; Showa Yakuhin Kako Co., Tokyo, Japan).8,9 The threads were placed in the medial canthus for 1 minute and the length of the wetted section was measured in millimeters. Corneal sensation was measured using a Cochet-Bonnet filament esthesiometer in unanesthetized rats (Luneau Ophtalmogie, France).8,9 The testing began with the nylon filament extended to the maximal length (6 cm). The end of the nylon filament was touched to the cornea. If the rat blinked (positive response) the length of the filament was recorded. If the rat did not blink then the nylon filament was shortened by 0.5 cm and the test repeated until a positive response was recorded. This process was repeated for each eye three times.
Motor and Sensory Nerve Conduction Velocity
On the day of terminal studies rats were weighed and anesthetized with Nembutal (50 mg/kg IP, Abbott Laboratories, North Chicago, IL). Motor nerve conduction velocity was determined as previously described using a noninvasive procedure in the sciatic-posterior tibial conducting system.12,15 Sensory nerve conduction velocity was determined using the digital nerve as described by Obrosova et al.16 Motor and sensory nerve conduction velocity was reported in meters per second.
Corneal Innervation
Subbasal corneal nerves were imaged using the Rostock cornea module of the Heidelberg Retina Tomograph confocal microscope as previously described.9 The anesthetized rat was secured to a platform that allowed adjustment and positioning of the rat in three dimensions. A drop of GenTeal (lubricant eye gel; Novartis Pharmaceutical Corp., East Hanover, NJ) was applied onto the tip of the lens and advanced slowly forward until the gel contacted the cornea allowing optical but not physical contact between the objective lens and corneal epithelium.3 Ten random high-quality images without overlap of the subbasal nerve plexus around the perimeter of the central cornea were acquired by finely focusing the objective lens to maximally resolve the nerve layer just under the corneal epithelium. It has been recently reported in human studies that acquiring eight random images not overlapping by more than 20% is a sufficient sample size for quantification of corneal subbasal nerve parameters.17 The investigator acquiring these images was masked with respect to identity of the animal condition. After acquisition of these images, the total corneal thickness of the central cornea was determined. For these studies a single parameter of corneal innervation was quantified. Corneal nerve fiber length defined as the total length of all nerve fibers and branches (in millimeters) present in the acquired images standardized for area of the image (in square millimeters) was determined for each image by tracing the length of each nerve in the image, summing the total length and dividing by the image area.3,8,9 The corneal fiber length for each animal was the mean value obtained from the 10 acquired images and expressed as millimeters per square millimeter. Based on receiver operating characteristic (ROC) curve analysis corneal nerve fiber length is the optimal parameter for diagnosing patients with diabetic neuropathy and has the lowest coefficient of variation.3,4 Other parameters commonly determined in human studies are corneal nerve fiber density, corneal nerve branch density, corneal nerve fiber width, and corneal nerve fiber tortuosity.3,4,6,18–20 However, differences in sampling and quantification of these parameters suggest caution when interpreting findings and the need for more studies to determine the most accurate sampling method for these other corneal parameters.20 In rats we have observed little corneal nerve fiber branching and have not tested the other parameters.
Additional measurements included nonfasting blood glucose and hemoglobin A1C levels (Glyco-tek affinity column; Helena Laboratories, Beaumont, TX). Serum was collected for determining levels of free fatty acid, triglyceride, free cholesterol, and angiotensin converting enzyme activity, using commercial kits from Roche, Sigma Chemical Co., Bio Vision (Mountain View, CA), and ALPCO Diagnostics (Salem, NH), respectively. Serum thiobarbituric acid reactive substances levels were determined as a marker of oxidative stress as previously described.12,14,15
Vascular Reactivity in Posterior Ciliary Artery
The ophthalmic artery travels along the inferior side of the optic nerve sheath and divides into three branches: the central retinal artery and medial and lateral posterior ciliary arteries. We isolated both ciliary arteries for vascular studies using videomicroscopy by carefully removing the entire eye along with the optic nerve and trimming excess tissue from the desired vessels.9,12 The isolated vessels were cannulated at both ends with glass micropipettes filled with buffer (4°C) and secured with 10-0 nylon monofilament sutures (Ethicon, Cornelia, GA). The pipettes were then attached to a single pressure reservoir (initially set at 0 mm Hg) under condition of no flow. The organ chamber containing the cannulated vessels was then transferred to the stage of an inverted microscope (CK2; Olympus, Lake Success, NY) as previously described.9,12 The organ chamber was connected to a rotary pump (Masterflex; Cole Parmer Instrument, Vernon Hills, IL), which continuously circulated 37°C oxygenated Krebs-Henseleit physiological saline solution (PSS) of the following composition: NaCl 118 mM, KCl 4.7 mM, CaCl2 2.5 mM, KH2PO4 1.2 mM, MgSO4 1.2 mM, NaHCO3 20 mM, Na2EDTA 0.026 mM, and glucose 5.5 mM at 30 mL/min. The pressure within the vessel was then slowly increased to 60 mm Hg. At this pressure, we found that KCl gave the maximal constrictor response. Therefore, all of the studies were conducted at 60 mm Hg. Internal vessel diameter (resolution of 2 μm) was measured by manually adjusting the video micrometer. After a 30-minute equilibration, KCl was added to the bath to test vessel viability. Vessels failing to constrict by at least 30% were discarded. After they were washed with PSS, vessels were incubated for 30 minutes in PSS and then constricted with phenylephrine (10−6 M; Cayman Chemical, Ann Arbor, MI) to 30% to 50% of passive diameter. Afterwards, a cumulative concentration–response relationships was evaluated for acetylcholine (10−8–10−4 M). At the end of each dose response curve papaverine (10−5 M) was added to determine maximal vasodilation. Data was presented as percent relaxation.
Intraepidermal Nerve Fiber Density in the Hindpaw and Innervation of the Epithelium of the Cornea
Immunoreactive nerve fiber profiles innervating the skin from the hindpaw and epithelium of the cornea, which are primarily sensory nerves, were visualized using confocal microscopy. Samples of skin of the right hindpaw and strips of the medial cornea extending across the entire diameter of the cornea were fixed, dehydrated, and embedded in paraffin. Three sections (7 μm for skin and 10 μm for cornea) for each animal were collected and immunostained with anti-PGP9.5 antibody (rabbit anti-human, AbD Serotic; Morpho Sys US Inc., Raleigh, NC) overnight followed by treatment with secondary antibody Alexa Fluor 546 goat anti-rabbit (Invitrogen, Eugene, OR). Profiles were counted by two individual investigators that were blinded to the sample identity. All immunoreactive profiles were counted and normalized to length.12,21
Data Analysis
Results are presented as mean ± SEM. Comparisons between the control and diabetic untreated and treated rats were conducted using a one-way ANOVA and Bonferroni-Dunn test for multiple comparisons (Prism software; GraphPad, San Diego, CA). Concentration–response curves for acetylcholine was compared using a two-way repeated measures ANOVA with autoregressive covariance structure using proc mixed program of SAS (Statistical Analysis System, SAS Institute Inc., Cary, NC).9,21 A P value of less than 0.05 was considered significant.
Results
Effect of Streptozotocin-Diabetes and Treatment with Ilepatril on Weight, Blood Glucose and Serum Lipid, and Thiobarbituric Acid Reactive Substances
Data in Table 1 demonstrate that diabetic rats continued to gain weight after the induction of diabetes, but this was significantly reduced compared to control rats. Treating diabetic rats with Ilepatril did not improve weight gain. Blood glucose levels were significantly increased in untreated and treated diabetic rats compared to control rats as were hemoglobin A1C levels. Data in Table 2 demonstrate that diabetic rats were hyperlipidemic, with serum triglycerides, free fatty acids, and free cholesterol levels all significantly elevated above control. Serum thiobarbituric acid reactive substances were also significantly increased in diabetic rats. Treating diabetic rats with Ilepatril significantly decreased serum triglyceride and cholesterol levels. In contrast serum free fatty acid levels and thiobarbituric acid reactive substances remained elevated in Ilepatril-treated diabetic rats. Angiotensin converting enzyme activity in the serum was increased by diabetes and significantly reduced with Ilepatril treatment.
Table 1. .
Change in Body Weight, Blood Glucose, and Hemoglobin A1C in Streptozotocin-Induced Diabetic Rats
|
Determination |
Control (9) |
Diabetic (10) |
Diabetic + Ilepatril (10) |
| Start weight, g | 337 ± 4 | 337 ± 3 | 338 ± 2 |
| End weight, g | 445 ± 6 | 357 ± 14* | 360 ± 16* |
| Blood glucose, mg/dL | 141 ± 5 | 584 ± 13* | 539 ± 27* |
| Hb A1C, % | 7.3 ± 0.5 | 14.3 ± 1.8* | 12.4 ± 1.0* |
Data are presented as the mean ± SEM. *P < 0.05 compared to control. Parentheses indicate the number of experimental animals.
Table 2. .
Change in Serum Thiobarbituric Acid Reactive Substances, Triglycerides, Free Fatty Acids, Cholesterol, and Angiotensin Converting Enzyme Activity and Corneal Sensitivity, Tear Production and Cornea Thickness in Streptozotocin-Induced Diabetic Rats
|
Determination |
Control (9) |
Diabetic (10) |
Diabetic + Ilepatril (10) |
| Thiobarbituric acid reactive substances, μg/mL | 0.78 ± 0.04 | 0.94 ± 0.04* | 0.92 ± 0.06 |
| Triglycerides, mg/dL | 14 ± 1 | 130 ± 16* | 52 ± 7+ |
| Free fatty acids, mmol/L | 0.06 ± 0.01 | 0.42 ± 0.06* | 0.46 ± 0.08* |
| Cholesterol, mg/mL | 1.2 ± 0.1 | 4.3 ± 0.5* | 1.7 ± 0.2+ |
| ACE activity, mU/mL | 34 ± 2 | 55 ± 6* | 17 ± 2*,+ |
| Corneal sensitivity, cm | 5.97 ± 0.03 | 5.77 ± 0.07* | 6.00 ± 0.00+ |
| Tear production, fiber length, mm | 22.3 ± 0.7 | 15.2 ± 0.8* | 17.5 ± 0.6* |
| Cornea thickness, μm | 137 ± 10 | 126 ± 6 | 121 ± 6 |
Data are presented as the mean ± SEM. One unit of angiotensin converting enzyme activity is defined as the amount of enzyme required to release 1 μmol of hippuric acid per minute and per liter of serum at 37°C. *P < 0.05 compared to control, +P < 0.05, compared to diabetic. Parentheses indicate the number of experimental animals.
Effect of Streptozotocin-Diabetes and Treatment with Ilepatril on Nerve Conduction Velocity, Thermal Nociception, and Intraepidermal Nerve Fiber Density
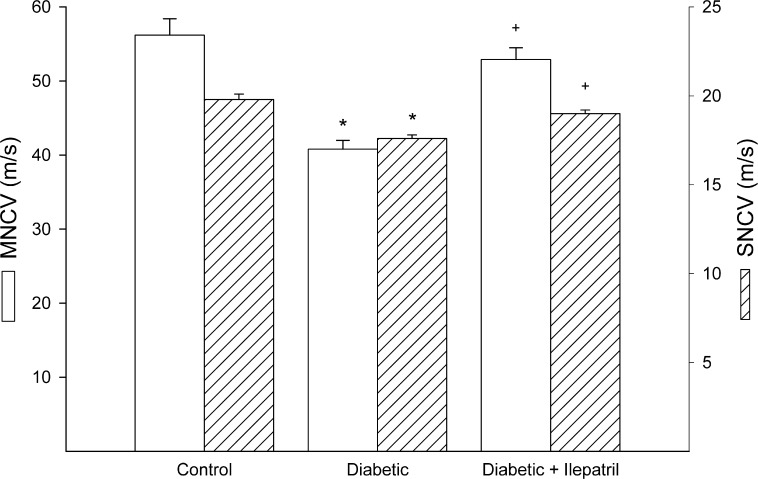
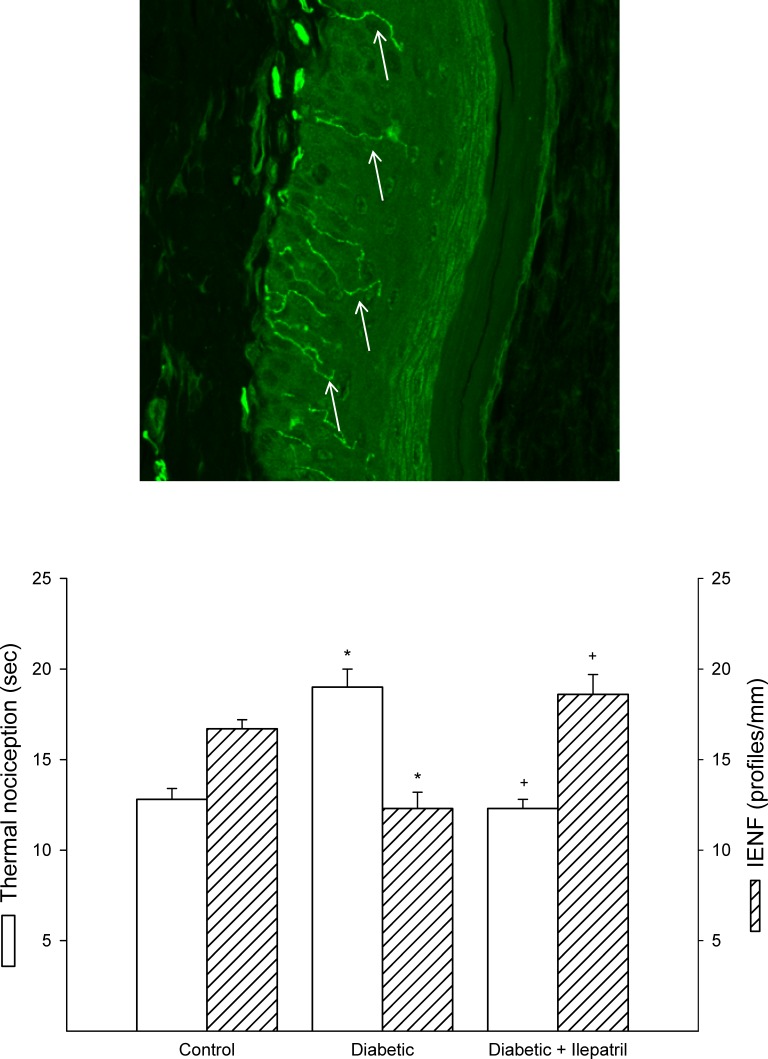
As we previously reported type 1 diabetes caused a significant decrease in motor and sensory nerve conduction velocities and this was significantly improved by Ilepatril treatment (Fig. 1).10,11 Type 1 diabetic rats after 8 weeks of diabetes were also thermal hypoalgesic and innervation of the epidermis was decreased (Fig. 2). Both of these deficits were prevented by treatment with Ilepatril.10,11
Figure 1. .
Effect of streptozotocin-diabetes and treatment with Ilepatril on motor and sensory nerve conduction velocity. Motor and sensory nerve conduction velocity was examined as described in the Methods section. Data are presented as the mean ± SEM in meters per second. The number of rats in each group was the same as shown in Table 1. *P < 0.05 compared to control rats; +P < 0.05 compared to diabetic rats.
Figure 2. .
Effect of streptozotocin diabetes and treatment with Ilepatril on thermal nociception and intraepidermal nerve fiber density. Top: Representative image of intraepidermal nerve fibers (white arrows) in skin from the hindpaw from a control rat. Bottom: Thermal nociception and intraepidermal nerve fiber density was examined as described in the Methods section. Data are presented as the mean ± SEM for thermal nociception in seconds and intraepidermal nerve fiber profiles per millimeter. The number of rats in each group was the same as shown in Table 1. *P < 0.05 compared to control rats; +P < 0.05 compared to diabetic rats.
Effect of Streptozotocin-Diabetes and Treatment with Ilepatril on Corneal Sensitivity and Innervation
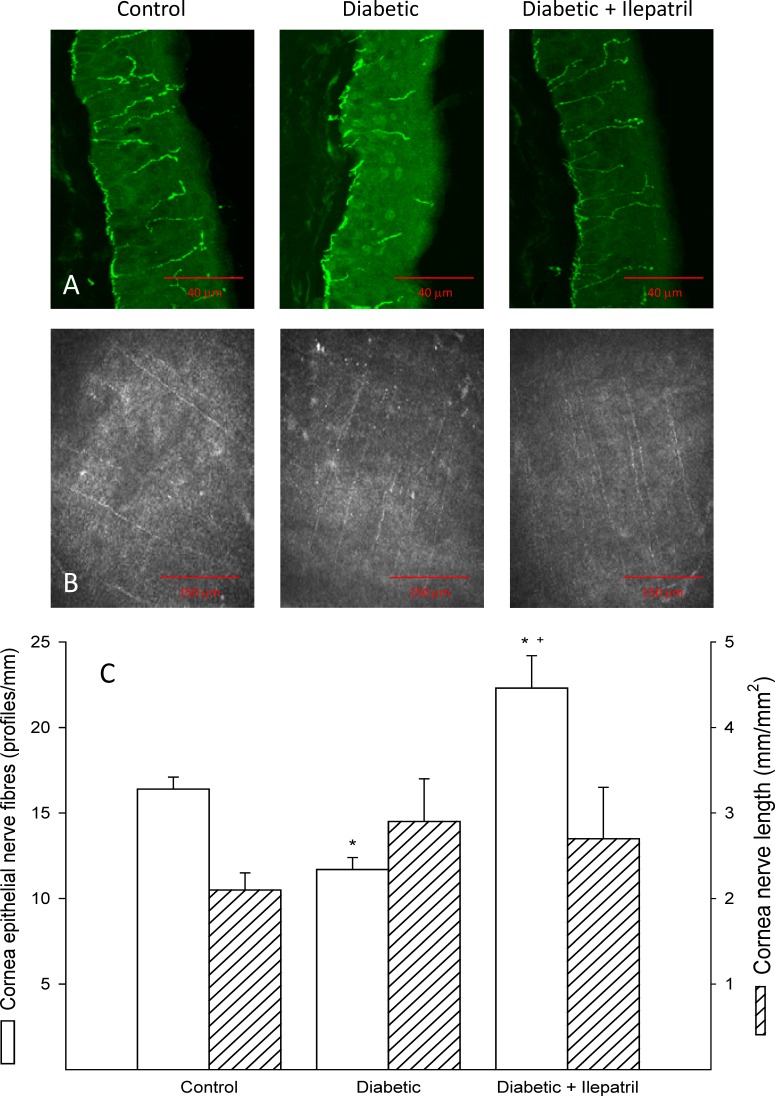
Figure 3 provides representative illustrations of nerve fibers innervating corneal epithelium (Fig. 3A) and the subbasal layer (Fig. 3B) from control, diabetic, and Ilepatril-treated diabetic rats. After 8 weeks of diabetes, innervation of the corneal epithelium was significantly decreased (Fig. 3C); whereas, subbasal corneal nerves were not impaired. Treating diabetic rats with Ilepatril significantly increased innervation of the corneal epithelium, and the increase was even significantly greater than in control rats. Tear secretion was significantly decreased in diabetic rats compared to control rats and this was not improved by treating diabetic rats with Ilepatril (Table 2). Corneal sensitivity as determined using Cochet-Bonnet filament esthesiometer was significantly decreased in diabetic rats compared to control rats, and this was completely prevented by treating diabetic rats with Ilepatril (Table 2). In this study a positive blinking response to the 6-cm-long filament (most sensitive) was obtained in 7 of 9 control rats, 4 of 10 diabetic rats, and 10 of 10 diabetic rats treated with Ilepatril. Corneal thickness was not changed by diabetes of 8 weeks' duration (Table 2).
Figure 3. .
Effect of streptozotocin-diabetes and treatment with Ilepatril on innervation of corneal epithelium and subbasal layer of the cornea. Representative images of nerves innervating corneal epithelium (A) and subbasal layer of the cornea (B) from control (left), diabetic (center), and diabetic treated with Ilepatril (right) rats. Innervation of the epithelium of the cornea was determined by immunohistochemical staining and subbasal layer by corneal confocal microscopy as described in the Methods section and the quantified data presented in C. Data are presented as the mean ± SEM. The number of rats in each group was the same as shown in Table 1. *P < 0.05 compared to control rats; +P < 0.05 compared to diabetic rats.
Effect of Streptozotocin-Diabetes and Treatment with Ilepatril on Acetylcholine-Mediated Vascular Relaxation by Posterior Ciliary Arteries
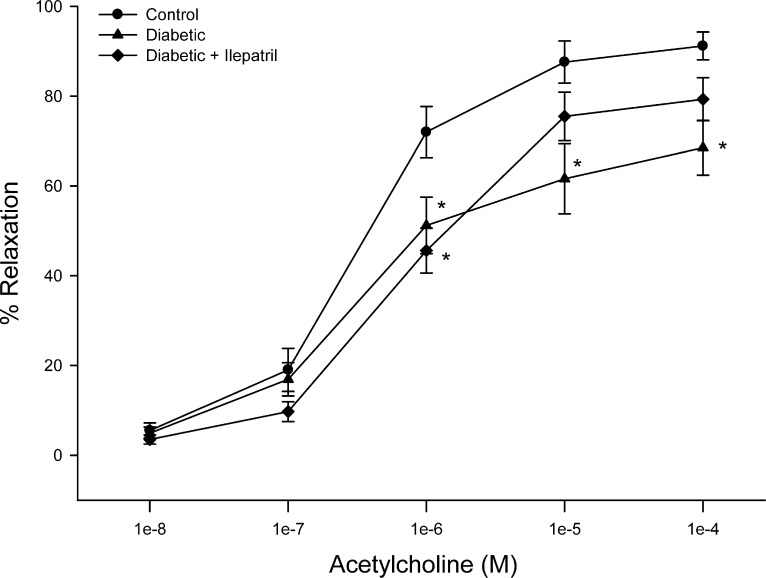
We have previously demonstrated that high fat/streptozotocin-induced diabetes caused a decrease in acetylcholine-mediated vascular relaxation in posterior ciliary arteries.9 Figure 4 demonstrates that acetylcholine-mediated vascular relaxation in posterior ciliary arteries was also impaired in type 1 diabetic rats after 8 weeks of hyperglycemia. Treating diabetic rats with Ilepatril for 6 weeks partially improved acetylcholine-mediated vascular relaxation.
Figure 4. .
Effect of streptozotocin-diabetes and treatment with Ilepatril on vascular relaxation by acetylcholine in posterior ciliary arteries. Pressurized vessels (60 mm Hg and ranging from 180 to 250 μm luminal diameters) were constricted with phenylephrine (30%–50%) and incremental doses of acetylcholine were added to the bathing solution while recording steady state vessel diameter. Data are presented as the mean of percent relaxation ± SEM. The number of rats in each group was the same as shown in Table 1. *P < 0.05 compared to control rats.
Discussion
Corneal confocal microscopy, a noninvasive in vivo imaging procedure that can be performed repeatedly, provides an assessment of subbasal corneal sensory nerve structure, and can detect early nerve damage in diabetic patients.3,6,22,23 It has been proposed that imaging of diabetes-induced changes of these nerves as well as measurement of corneal sensitivity may be used as surrogate markers for diabetic neuropathy.4,6,20,24
An important result in this study was the finding that innervation of corneal epithelium was decreased prior to a detectable decrease of subbasal corneal nerves. If this would also occur in humans it could have major clinical implications, suggesting that development of methodology to image in vivo corneal nerves in the epithelium may provide an earlier endpoint for the evaluation of development and treatment of diabetic neuropathy than confocal imaging of subbasal corneal nerves.25 The corneal epithelium is one of the most densely innervated superficial tissues in the human body with an estimated 7000 nociceptors/mm2.25,26 These nociceptors activate corneal reflex in response to mechanical, chemical, and temperature stimuli.25 The cornea is innervated by myelinated Aδ- and unmyelinated C-fibers. Aδ- and C-fibers can be clearly distinguished by fiber diameter and, while running in the basal epithelial plexus, by their spatial arrangement.25,27
The decrease in innervation of corneal epithelium of diabetic rats was mirrored by a decrease in corneal sensitivity as measured by Cochet-Bonnet filament esthesiometer and tear secretion and intraepidermal nerve fiber density in the skin of the hindpaw as well as impairment of other standard endpoints for diabetic neuropathy. Assessing intraepidermal nerve fiber pathology has been shown to be useful in identifying patients with small fiber neuropathy.28 However, this is an invasive procedure that requires a high degree of expertise to reproducibly process and quantify. Imaging subbasal corneal nerves and perhaps corneal nerves of the epithelium may provide an improved approach for assessing small nerve fiber pathology that would be better tolerated by patients, especially if repeated sampling is required.
After 8 weeks of diabetes we did not observe a decrease of subbasal corneal nerves. This outcome is different from a similar study we performed with rats fed a high fat diet and treated with low-dose streptozotocin as a model for type 2 diabetes.9 However, in those studies rats were fed a high fat diet for 20 weeks and were hyperglycemic for the final 12 weeks of the 20-week period. Therefore, the duration of hyperglycemia was longer in the studies using type 2 diabetic rats than in these studies.9 The type 2 diabetic rats used in our previous study were also hypertensive. Whether elevated blood pressure contributes to corneal nerve impairment in humans is disputed after two studies from the same group of investigators obtained differing results that may be dependent on severity of diabetic neuropathy of patients upon entry into the study.5,18 It should also be noted that the type 2 diabetic rats in our study were extremely insulin resistant.9 In our present study the type 1 diabetic rats were treated daily with a low dose of insulin in order to maintain body weight. The dose of insulin used did not correct hyperglycemia. However, the low dose of insulin could have affected corneal nerve fiber damage. Neurons express insulin receptors, and it has been shown that insulin can improve nerve function.29 Another caveat to keep in mind is the relatively small number of animals studied compared to most human studies. It could be that a larger sample size may provide a statistical difference. Overall, these factors could contribute to the difference in corneal nerve damage as assessed by corneal confocal microscopy observed in our studies of type 1 and type 2 diabetic rats.
Yin et al.8 using streptozotocin-induced diabetic rats of 8 weeks' duration demonstrated decreased tear secretion, attenuated corneal sensitivity, and reduced subbasal innervation. Corneal thickness was unchanged and innervation of the epithelium was not examined. Our results are consistent with those of Yin et al.8 except for reduced subbasal corneal innervation. Even though both studies were performed using Sprague-Dawley rats after 8 weeks of hyperglycemia there are a number of differences in the design of the two studies that may explain why unlike Yin et al.8 we did not observe a decrease in subbasal corneal innervation. The studies by Yin et al.8 were initiated with 6-week-old rats compared to our studies using rats that were 12 weeks of age. In younger rats nerves are still developing and growing. Inducing diabetes at a younger age may arrest nerve development contributing to the appearance of nerve degeneration. In addition, to induce diabetes Yin et al.8 used two daily doses of 40 mg/kg streptozotocin compared to our single injection of 55 mg/kg. Lastly, we treated our diabetic rats with a low dose of insulin daily. Even though the dose of insulin did not correct hyperglycemia it could have provided some degree of nerve protection, and it is possible that a longer duration of diabetes using our protocol will be necessary to observe a decrease in subbasal innervation of the cornea.
The posterior ciliary artery is the main source of blood supply to trigeminal nerves in the orbit, cornea, and the optic nerve head, and it also supplies the choroid up to the equator, the retinal pigment epithelium, the outer portion of the retina, and the medial and lateral segments of the ciliary body and iris. That makes the posterior ciliary artery the most important part of the ocular and optic nerve head circulation. Disturbances in the posterior ciliary artery circulation could contribute to a variety of ocular and vascular disorders.30 In the rat, vascular reactivity of the posterior ciliary artery is mediated by peptidergic, adrenergic, and nitrergic innervation/mechanisms.31 Even though the cornea is avascular the posterior ciliary artery provides important circulation to cornea support tissues and is the ideal vessel to study the impact of diabetes on vascular function as it relates to the eye. We had previously demonstrated that acetylcholine-mediated vascular relaxation of the posterior ciliary artery was impaired in type 2 diabetic rats. We have now obtained similar results in a rat model of type 1 diabetes. Acetylcholine-mediated vascular relaxation by the posterior ciliary artery is due primarily to the generation of nitric oxide.9 This makes the posterior ciliary artery very susceptible to oxidative stress. Superoxide quenches nitric oxide resulting in the formation of peroxynitrite. We have previously demonstrated that the generation of superoxide is responsible for decreasing acetylcholine-mediated vascular relaxation by epineurial arterioles from diabetic rats.32 Future studies will explore whether this mechanism is responsible for impairing vascular reactivity in the posterior ciliary artery.
Another major finding of these studies was that treating diabetic rats with Ilepatril prevented the decreased innervation of corneal nerves into the corneal epithelium and improved corneal sensitivity as measured by Cochet-Bonnet filament esthesiometer but did not significantly improve tear secretion. Treatment of diabetic rats with Ilepatril also improved acetylcholine-mediated vascular relaxation by posterior ciliary arteries. Previously in studies of patients with diabetic neuropathy corneal nerve morphology was improved by improving associated risk factors.5 Corneal nerve regeneration has also been demonstrated in diabetic patients following pancreas transplantation.19 However, this is the first study to demonstrate that a pharmacological treatment in an animal model of diabetes can provide protection of corneal nerve structure and function. We have previously demonstrated that treating diabetic rats with Ilepatril, a vasopeptidase inhibitor, is a very effective treatment for diabetic neuropathy as determined by measuring the standard endpoints and vascular reactivity of epineurial arterioles.2,10–12 Vasopeptidase inhibitors block angiotensin converting enzyme and neural endopeptidase.2 We have attributed the success of this drug to its ability to block the adverse effects of angiotensin II and protect vaso- and neuro-active peptides from degradation by neutral endopeptidase.2 In humans angiotension converting enzyme and angiotensin II were found in endothelial and epithelial cells of the cornea.33 Hyperactivity of the renin-angiotensin system induces inflammation and retinal neural dysfunction associated with diabetes and blockade of this system has therapeutic implications for diabetic retinopathy.34,35 Expression of neutral endopeptidase is increased by diabetes and we have detected neutral endopeptidase activity in the cornea of control rats (21.5 ± 1.9 μM amido methyl coumarin/min/mg protein [n = 5]).10,12 Whether neutral endopeptidase is increased by diabetes in the cornea will be determined.
In summary, these studies have demonstrated that decreased innervation of the corneal epithelium occurs early in diabetic rats and is accompanied by a decrease in corneal sensitivity and precedes changes in nerve morphometry of the subbasal layer of the cornea. These studies have also shown that treatment of diabetic rats with Ilepatril prevents and/or improves many of the corneal and vascular changes associated with early diabetes.
Footnotes
Supported in part by material based upon work by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Biomedical Laboratory Research and Development (RX000889-01) and by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK073990 from the National Institutes of Health. The content of this manuscript is new and solely the responsibility of the authors and do not necessarily represent the official views of the granting agencies. We would also like to extend our appreciation to Sanofi Aventis for supplying Ilepatril for these studies.
Disclosure: E.P. Davidson, None; L.J. Coppey, None; M.A. Yorek, None
References
- 1.Tesfaye S, Selvarajah D. The Eurodiab study: what has this taught us about diabetic peripheral neuropathy? Curr Diab Rep. 2009;9:432–434 [DOI] [PubMed] [Google Scholar]
- 2.Yorek MA. The potential role of angiotensin converting enzyme and vasopeptidase inhibitors in the treatment of diabetic neuropathy. Curr Drug Targets. 2008;9:77–84 [DOI] [PubMed] [Google Scholar]
- 3.Quattrini C, Tavakoli M, Jeziorska M, et al. Surrogate markers of small fiber damage in human diabetic neuropathy. Diabetes. 2007;56:2148–2154 [DOI] [PubMed] [Google Scholar]
- 4.Tavakoli M, Quattrini C, Abbott C, et al. Corneal confocal microscopy a novel noninvasive test to diagnose and stratify the severity of human diabetic neuropathy. Diabetes Care. 2010;33:1792–1797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tavakoli M, Kallinikos P, Iqbal A, et al. Corneal confocal microscopy detects improvement in corneal nerve morphology with an improvement in risk factors for diabetic neuropathy. Diabet Med. 2011;28:1261–1267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Malik RA, Kallinikos P, Abbott CA, et al. Corneal confocal microscopy: a non-invasive surrogate of nerve fibre damage and repair in diabetic patients. Diabetologia. 2003;46:683–688 [DOI] [PubMed] [Google Scholar]
- 7.Tavakoli M, Hossain P, Malik RA. Clinical applications of corneal confocal microscopy. Clin Ophthalmol. 2008;2:435–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yin J, Huang J, Chen C, Gao N, Wang F, Yu FS. Corneal complications in streptozotocin-induced type 1 diabetic rats. Invest Ophthalmol Vis Sci. 2011;52:6589–6596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davidson EP, Coppey LJ, Holmes A, Yorek MA. Changes in corneal innervation and sensitivity and acetylcholine-mediated vascular relaxation of the posterior ciliary artery in a type 2 diabetic rat. Invest Ophthalmol Vis Sci. 2012;53:1182–1187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oltman CL, Davidson EP, Coppey LJ, Kleinschmidt TL, Dake B, Yorek MA. Role of the effect of inhibition of neutral endopeptidase on vascular and neural complications in streptozotocin-induced diabetic rats. Eur J Pharmacol. 2011;650:556–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davidson EP, Kleinschmidt TL, Oltman CL, Lund DD, Yorek MA. Treatment of streptozotocin-induced diabetic rats with AVE7688, a vasopeptidase inhibitor, effect on vascular and neural disease. Diabetes. 2007;56:355–362 [DOI] [PubMed] [Google Scholar]
- 12.Davidson EP, Coppey LJ, Holmes A, Dake B, Yorek MA. Effect of treatment of high fat fed/low dose streptozotocin-diabetic rats with Ilepatril on vascular and neural complications. Eur J Pharmacol. 2011;688:497–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Calcutt NA. Modeling diabetic sensory neuropathy in rats. Methods Mol Med. 2004;99:55–65 [DOI] [PubMed] [Google Scholar]
- 14.Oltman CL, Davidson EP, Coppey LJ, Kleinschmidt TL, Lund DD, Yorek MA. Attenuation of vascular/neural dysfunction in Zucker rats treated with enalapril or rosuvastatin. Obesity. 2008;16:82–89 [DOI] [PubMed] [Google Scholar]
- 15.Coppey LJ, Davidson EP, Dunlap J, Lund DD, Yorek MA. Slowing of motor nerve conduction velocity in streptozotocin-induced diabetic rats is preceded by impaired vasodilation in arterioles that overlie the sciatic nerve. Int J Exp Diabetes Res. 2000;1:131–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Obrosova IG, Li F, Abatan OI, et al. Role of poly(ADP-ribose) polymerase activation in diabetic neuropathy. Diabetes. 2004;53:711–720 [DOI] [PubMed] [Google Scholar]
- 17.Vagenas D, Pritchard N, Edwards K, et al. Optimal image sample size for corneal nerve morphometry. Optom Vis Sci. 2012;89:812–817 [DOI] [PubMed] [Google Scholar]
- 18.Edwards K, Pritchard N, Vagenas D, Russell A, Malik RA, Efron N. Utility of corneal confocal microscopy for assessing mild diabetic neuropathy: baseline findings of the LANDMark study. Clin Exp Optom. 2012;95:348–354 [DOI] [PubMed] [Google Scholar]
- 19.Mehra S, Tavakoli M, Kallinikos PA, et al. Corneal confocal microscopy detects early nerve regeneration after pancreas transplantation in patients with type 1 diabetes. Diabetes Care. 2007;30:2608–2612 [DOI] [PubMed] [Google Scholar]
- 20.Pritchard N, Edwards K, Shahidi AM, et al. Corneal markers of diabetic neuropathy. Ocul Surf. 2011;9:17–28 [DOI] [PubMed] [Google Scholar]
- 21.Terata K, Coppey LJ, Davidson EP, Dunlap JA, Gutterman DD, Yorek MA. Acetylcholine-induced arteriolar dilation is reduced in streptozotocin-induced diabetic rats with motor nerve dysfunction. Br J Pharmacol. 1999;128:837–843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hossain P, Sachdev A, Malik RA. Early detection of diabetic peripheral neuropathy with corneal confocal microscopy. Lancet. 2005;366:1340–1343 [DOI] [PubMed] [Google Scholar]
- 23.Quattrini C, Tavakoli M, Jeziorska M, et al. Surrogate markers of small fiber damage in human diabetic neuropathy. Diabetes. 2007;56:2148–2154 [DOI] [PubMed] [Google Scholar]
- 24.Pritchard N, Edwards K, Vagenas D, Russell AW, Malik RA, Efron N. Corneal sensitivity is related to established measures of diabetic peripheral neuropathy. Clin Exp Optom. 2012;95:355–361 [DOI] [PubMed] [Google Scholar]
- 25.Guthoff RF, Wienss H, Hahnel C, Wree A. Epithelial innervation of human cornea: a three-dimensional study using confocal laser scanning fluorescence microscopy. Cornea. 2005;24:608–613 [DOI] [PubMed] [Google Scholar]
- 26.Muller LJ, Marfurt CF, Kruse F, Tervo TM. Corneal nerves: structure, contents and function. Exp Eye Res. 2003;76:521–542 [DOI] [PubMed] [Google Scholar]
- 27.Tavakoli M, Petropoulos IN, Malik RA. Assessing corneal nerve structure and function in diabetic neuropathy. Clin Exp Optom. 2012;95:338–347 [DOI] [PubMed] [Google Scholar]
- 28.Malik RA. Early detection of nerve damage and repair in diabetic neuropathy. Nat Clin Pract Neurol. 2008;4:646–647 [DOI] [PubMed] [Google Scholar]
- 29.Brussee V, Cunningham FA, Zochodne DW. Direct insulin signaling of neurons reverses diabetic neuropathy. Diabetes. 2004;53:1824–1830 [DOI] [PubMed] [Google Scholar]
- 30.Hayreh S. Posterior ciliary artery circulation in health and disease: the Weisenfeld lecture. Invest. Opthamol Vis Sci. 2004;45:749–757 [DOI] [PubMed] [Google Scholar]
- 31.Bergua A, Schrodl F, Neuhuber WL. Vasoactive intestinal and calcitonin gene-related peptides, tyrosine hydroxylase and nitrergic markers in the innervation of the rat central retinal artery. Exp Eye Res. 2003;77:367–374 [DOI] [PubMed] [Google Scholar]
- 32.Coppey LJ, Gellett JS, Davidson EP, Dunlap JA, Lund DD, Yorek MA. Effect of antioxidant treatment of streptozotocin-induced diabetic rats on endoneurial blood flow, motor nerve conduction velocity, and vascular reactivity of epineurial arterioles of the sciatic nerve. Diabetes. 2001;50:1927–1937 [DOI] [PubMed] [Google Scholar]
- 33.Savaskan E, Loffler KU, Meier F, Muller-Spahn F, Flammer J, Meyer P. Immunohistochemical localization of angiotensin-converting enzyme, angiotensin II and AT1 receptor in human ocular tissues. Ophthalmic Res. 2004;36:312–320 [DOI] [PubMed] [Google Scholar]
- 34.Kurihara T, Ozawa Y, Ishida S, Okano H, Tsubota K. Renin-angiotensin system hyperactivation can induce inflammation and retinal neural dysfunction. Int J Inflam. 2012;2012:581695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jeganathan VS. The therapeutic implications of renin-angiotensin system blockade in diabetic retinopathy. Curr Pharm Biotechnol. 2011;12:392–395 [DOI] [PubMed] [Google Scholar]