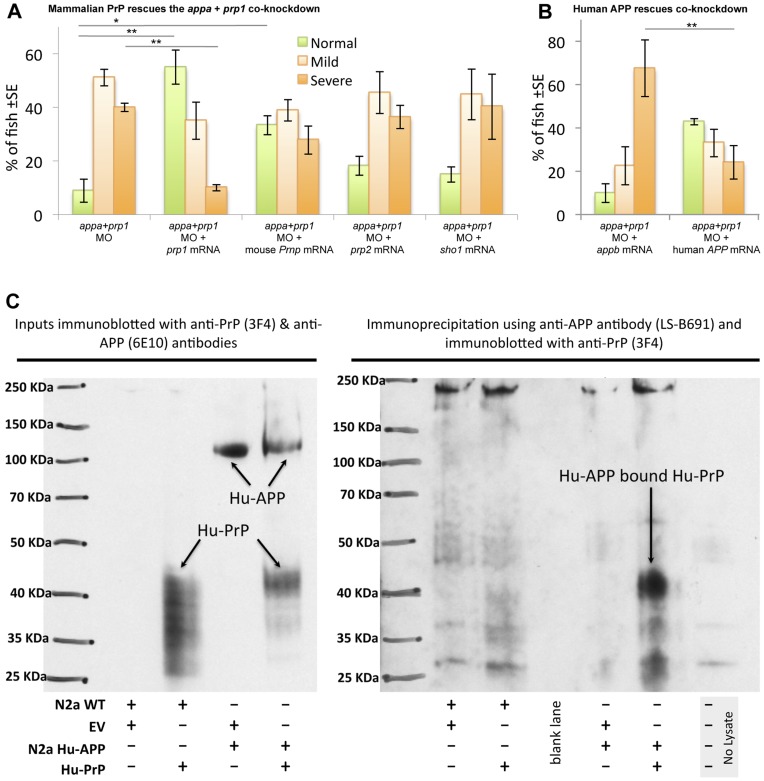
Figure 6. APP interactions with PrP are conserved from fish to mammals. A. Mouse Prnp can replace zebrafish prp1 in the context of its genetic interaction with appa.
Co-injecting zebrafish prp1 mRNA, in concert with the Appa+Prp1 co-knockdown, rescues the observed phenotypes (first two sets of bars). prp1’s paralog, zebrafish prp2, does not rescue this co-knockdown, nor does another prion family member from zebrafish, shadoo1. In contrast, mouse Prnp mRNA (moPrP) can partially alleviate the Appa & Prp1 co-knockdown. Thus mouse PrP can replace Prp1 in the context of its interaction with App, indeed with greater efficacy than zebrafish orthologs. * p<0.05. **p<0.01. B. Human APP can replace zebrafish appa in the context of its genetic interaction with prp1 . We established above that appa mRNA from zebrafish can rescue the co-knockdown of Appa+Prp1; Here we use APPb as a negative control comparator mRNA (see Fig. 3K). Human APP695 mRNA (huAPPwt) was effective in replacing zebrafish APPa in the context of Prp1 knockdown. C. Co-immunoprecipitation demonstrates an interaction between human PrP and human APP in N2a cells. Left: Inputs as whole cell lysate showing expression of human PrP using the human PrP specific antibody 3F4 in N2a cells (wild type and stably transfected with human APP) transiently transfected with pcDNA3-human PrP construct but not with empty vector (“EV”). Expression of human APP is only observed in N2a cells with human APP using 6E10 antibody, specific for human APP. Input represented 7% of whole cell lysate used for co-immunoprecipitation. Right: whole cell lysates were co-immunoprecipitated using a human specific anti-APP antibody followed by immunoblotting with a human PrP specific antibody. Detection of human APP bound human PrP was observed only in N2a cells stably transfected with human APP and transiently transfected with human PrP construct. A no lysate immunoprecipitation experiment was included as an additional negative control.