Abstract
Regular interactions between commensal bacteria and the enteric mucosal immune environment are necessary for normal immunity. Alterations of the commensal bacterial communities or mucosal barrier can disrupt immune function. Chronic stress interferes with bacterial community structure (specifically, α-diversity) and the integrity of the intestinal barrier. These interferences can contribute to chronic stress-induced increases in systemic IL-6 and TNF-α. Chronic stress, however, produces many physiological changes that could indirectly influence immune activity. In addition to IL-6 and TNF-α, exposure to acute stressors upregulates a plethora of inflammatory proteins, each having unique synthesis and release mechanisms. We therefore tested the hypothesis that acute stress-induced inflammatory protein responses are dependent on the commensal bacteria, and more specifically, lipopolysaccharide (LPS) shed from Gram-negative intestinal commensal bacteria. We present evidence that both reducing commensal bacteria using antibiotics and neutralizing LPS using endotoxin inhibitor (EI) attenuates increases in some (inflammasome dependent, IL-1 and IL-18), but not all (inflammasome independent, IL-6, IL-10, and MCP-1) inflammatory proteins in the blood of male F344 rats exposed to an acute tail shock stressor. Acute stress did not impact α- or β- diversity measured using 16S rRNA diversity analyses, but selectively reduced the relative abundance of Prevotella. These findings indicate that commensal bacteria contribute to acute stress-induced inflammatory protein responses, and support the presence of LPS-mediated signaling in stress-evoked cytokine and chemokine production. The selectivity of the commensal bacteria in stress-evoked IL-1β and IL-18 responses may implicate the inflammasome in this response.
Introduction
The enteric mucosal immune system is a unique immunological site that must maintain a balance between responding to harmful pathogens and avoiding inappropriate immune responses to food or symbiotic bacteria. During a brief developmental period, ecological secession culminates in a relatively stable community of commensal bacteria [1]. Regular interactions between the mucosal immune system and these bacteria are critical for proper regulation of mucosal as well as systemic immune function [2]–[4]. Moreover, disruptions to the mucosal environment such as changes in barrier function or microbial composition can lead to severely dysregulated immunity [5], [6].
Several diverse factors may impact the mucosal barrier or the composition of the commensal bacteria including antibiotic use [7], [8], changes to diet or hygiene [7], [8], and activation of the stress response [9]–[14]. Dense sympathetic innervation of the intestine [15], and stress-inducible, localized mast cell degranulation [5], could facilitate stress-evoked changes to both the composition of the commensal bacteria [9], [11], [16], [17] and the integrity of the intestinal barrier [13], [18], [19]. Importantly, stress-induced changes to the intestinal barrier or the composition of the commensal bacteria appear to drive some aspects of stress-evoked mucosal and even systemic immune activity. Stress-induced disruptions to the mucosal barrier, for example, are linked to increased serum cytokine levels including tumor necrosis factor α (TNFα) [20]. Similarly, reducing the commensal bacteria via antibiotic administration attenuates chronic or repeated stress-induced enhancements in splenic macrophage activity [18] and circulating levels of the cytokine interleukin-6 (IL-6) [1].
Exposure to stressors, however, evokes a broad cytokine and chemokine response beyond the few cytokines that manipulations to the mucosal environment have been shown to modulate. Stress, for example, increases circulating concentrations of several inflammatory proteins including not only TNFα and IL-6, but also interleukin-1β (IL-1β) [21]–[23], interleukin-18 (IL-18) [21], interleukin-10 (IL-10) [24], and monocyte chemotactic protein-1 (MCP-1) [25]–[27]. Importantly, these and other cytokines operate in networks with other inflammatory proteins to achieve immunological effects [24]. Moreover, activation, synthesis, release, and mechanisms of various stress-responsive cytokines and chemokines are different and could vary in their modulation by the intestinal bacteria. Multiple stress-responsive cytokines must therefore be considered when investigating the role of intestinal bacteria in stress-induced alterations in immune activation.
Furthermore, previous studies implicating changes to the enteric mucosal immune system in stress-evoked immune activity focus on chronic or repeated stressors such as social defeat or repeated restraint [1]. These stressors not only activate the stress response, but can produce long-term changes to metabolic processes [28], feeding [29], and grooming behavior [30], which could themselves influence immune function or the role of intestinal bacteria in stress-evoked immune activation. Stress-evoked cytokine and chemokine secretion occurs in response to acute stressors. Thus the acute stress response itself might affect the production of these cytokines independent of other stress-evoked long-term adaptations. Understanding the role of commensal bacteria in the acute stress-induced production of a broad range of inflammatory proteins could provide important new information about how stress affects specific immunological pathways.
We therefore tested the hypothesis that acute stress-induced immune modulation depends on commensal bacteria. We reduced commensal bacteria using antibiotics, exposed rats to an acute tail shock stressor, and measured cytokine and chemokine production. Alterations in gut microbiota composition can influence immune function. A second goal was to test if exposure to an acute stressor would produce changes in microbiota diversity measured using 16S rRNA diversity analyses. Finally, the mechanism by which the commensal bacteria communicate with the immune system during stressor exposure, including acute stressor exposure, remains unknown. LPS, a microbe-associated molecular pattern (MAMP), is found in the cell membrane of some commensal bacteria and can increase in the circulation [19] following intestinal barrier disruption as occurs with chronic stress. Thus a third goal was to determine whether LPS is an important signaling molecule for communication between commensal bacteria and the immune system. We administered endotoxin inhibitor (EI) to block LPS, and measured circulating cytokines and chemokines after acute stress. Our results make several novel contributions to the literature in that they reveal an important role for intestinal bacteria in acute stress-induced immune activation, and support the presence of LPS-mediated signaling from the commensal bacteria in stress-induced cytokine and chemokine production. Furthermore, the results may reveal details of the signaling pathway underlying stress-evoked cytokine and chemokine production and could support the future development of therapeutics designed to manipulate stress-induced immune activity.
Methods
Subjects and Housing
Adult male Fischer 344 rats (240–260 g) were divided equally into four groups crossing stress and antibiotic (N = 64) or stress and EI administration (N = 32). The Fischer Rat is a highly stress responsive inbred rat, and was chosen for these experiments as the stress response is robust and consistent across animals allowing us to use fewer animals per group. To characterize the impact of stress on the commensal bacteria, rats (N = 25) were divided into 3 groups to examine the effect of stress both immediately and 24 hours following stressor termination. All rats were maintained on a 12∶12-h light-dark cycle (lights on from 0700 to 1900) in a specific pathogen free environment. Animals were allowed two weeks to acclimate to the colony room prior to any experimental manipulation. Rats were handled briefly each day for 1 week before the start of the study. All animals were housed in Plexiglas Nalgene cages and allowed ad libitum access to food (Harlan Laboratories, Denver, CO) and water. Colony room temperature was maintained at 23°C. The care and treatment of the animals were in accordance with protocols approved by the University of Colorado Institutional Animal Care and Use Committee.
Stress
On the day of the experiment, animals either remained in their home cages (Control) or were exposed to 100, 1.5 mA, 5-second, intermittent, (average trial interval = 60 seconds+/−25 seconds) inescapable tail shocks (Stress) as previously described [24], [35]–[37]. During the stress procedure, rats were placed in a Plexiglas restraining tube (23.4 cm long, 7 cm diameter). Electrodes were then placed across the tail that protruded from the back of the shock tube. The shocks were administered by an automated shock system (Precision Calculated Animal Shocker; Colbourne Instruments). This tail shock procedure is a well-established model of acute stress that has been thoroughly characterized in terms of both the stress response and the immune response. To examine the role of the commensal flora in an immune response to an acute stressor we selected this model of stress for its robust impact on immune function [24], [31]–[34]. Stress occurred between 0730 and 1130 to avoid differences in cytokine and chemokine production due to circadian rhythms. Immediately after termination of stress, all animals were sacrificed via rapid decapitation unless otherwise noted.
Quantification of the stress response
Because the duration, intensity, and chronicity of a stressor determines the immunological consequences of a stressor exposure [35], [38], [39], and because the absence of the commensal bacteria in germ-free rodents modulates HPA responses after stress [40], we measured two important markers of activation of the stress response to provide a characterization of tail shock. Corticosterone and spleen weights were measured to demonstrate activation of the stress response. Corticosterone is a measure of hypothalamic-pituitary-adrenal axis (HPA) output, and reductions in spleen weight are directly proportional to sympathetic nervous system activity. Corticosterone was measured in 96-well microtiter plates using commercially available ELISAs in accordance with manufacturer's instructions (Enzo Life Sciences). Optical densities were measured using a SpectraMax Plus 354 plate reader (Molecular Devices) and concentrations were analyzed using a four-parameter curve fitting software (SoftMax 5.4.1). Spleens were harvested aseptically and weighed immediately.
Antibiotic Administration
For 4 days prior to Stress rats received either drinking water plus 4.0 mg/ml streptomycin and 2.0 mg/ml penicillin g (antibiotic) or drinking water alone (water) ad libitum as previously described [41], [42]. Antibiotics were administered in the drinking water to avoid the potential stress-response associated with other delivery methods such as oral gavage [43], [44]. The current antibiotic regimen was selected for its broad-spectrum antibacterial effects and because it is consumed by the rats without the addition of any flavoring or sweetener. Each morning, antibiotic solution was replaced because penicillin G has a short half-life at room temperature. Water bottles were weighed daily to estimate water intake of all rats and ensure equivalent doses between animals. Body weights were recorded and fecal matter was examined to monitor sickness or diarrhea in rats receiving antibiotics.
Endotoxin Inhibitor Administration
On the day of Stress, EI was prepared by dissolving 1.0 mg/ml of EI into sterile PBS, which was stored on ice in the dark until use. Fifteen minutes prior to Stress, rats received an intraperitoneal injection (i.p.) of either 1.0 mg/kg EI (Bachem) or PBS alone. This dose was adapted from previous investigations demonstrating that this concentration of EI was sufficient to reduce LPS activity [45], [46]. The time between the injection of EI and the start of Stress was necessary to achieve maximal efficacy of the drug based upon the short half-life of EI.
Sample Collection
Immediately following sacrifice, whole blood was collected in EDTA coated vacutainers using a polypropylene funnel and centrifuged at 3000×g for 15 minutes at 4°C to obtain plasma samples. Fecal samples were collected from each animal immediately prior to the beginning of stress in sterile, media free, dual culture swabs (Becton Dickinson). Additional samples were taken immediately following termination of stress and from animals 24 hours following the termination of stress in the same manner. Following collection, all samples were frozen at −80°C.
Quantification of Bacteria
In order to confirm the efficacy of our antibiotic regimen, fresh fecal samples were collected from a subset of rats immediately prior to the beginning of stress. These samples were homogenized in 2.0 ml PBS and plated at several dilutions on nutrient agar. Plated samples were allowed to incubate at 37.0°C for 48 hours. Following incubation, colony forming units (CFU) of bacteria were counted, and dilution-corrected averages were recorded. Although many anaerobic bacteria will not grow on nutrient agar, this media was selected because it grows both Gram-positive and Gram-negative bacteria. Because the selected antibiotic regimen is broad spectrum, and targets both aerobic and anaerobic bacteria, reduced CFU counted on nutrient agar confirms effective reduction of commensal bacteria by the antibiotic regimen.
Endotoxin Measurement
In a separate experiment, whole blood was collected from Stress and Control rats in endotoxin free tubes. After 1 hr at room temperature, these samples were centrifuged at 3000×g for 15 min at 4.0°C to separate serum. LPS was quantified in serum using a Limulus amebocyte lysate (LAL) assay per manufacturer's instructions (Lonza).
16S rRNA microbial community analysis
Fecal samples and cecal contents were collected and prepared for sequencing using previously established protocols [47], [48]. Briefly, the MoBio 96 htp PCR clean up kit was used to triplicate, combine, and clean each sample. The samples underwent the PCR reaction with both forward and reverse primers (F515/R806) to target the V4 variable region of the 16S rRNA. The reverse primer contained an error-correcting 12-base Golay code allowing correct demultiplexing of ∼1,500 samples even when sequencing introduces errors in the barcode region. After gel purification and ethanol precipitation to remove PCR artifacts, a composite sample containing equimolar ratios of the amplicons were sequenced with the Illumina HiSeq 2000 (average sequences per sample 34,664±13,577 standard deviation (SD)). The open-source software package QIIME 1.3.0 [49] was used to process the sequences and conduct statistical analysis. Sequences were clustered into operational taxonomic units (OTUs) based on 97% sequence similarity using Uclust [50]. Taxonomy was assigned to OTUs using the Ribosomal Database Project classifier [51] against the GreenGenes 16S rRNA database [52].
Alpha diversity of samples was assessed with the Phlyogenetic Diversity metric, and results confirmed with Chao1 and observed species metrics (data not shown). Each sample was randomly subsampled 10 times, without replacement, at sequence depths from 2,000 to 30,000 sequences per sample at steps of 2,000 sequences per sample. The error bars on graphs depicting alpha diversity measures indicate the range of alpha diversity values at each sampling depth. ANOVA was used to determine whether any bacterial taxa significantly changed in abundance as a result of stress, with a p-value <0.05 following false discovery rate (FDR) correction.
Cytokine measurement
Circulating concentrations of cytokines (IL-1β, IL-6, IL-10, MCP-1, IL-18, IL-15) were measured from plasma in 96-well microtiter plates using commercially available ELISAs in accordance with the manufacturer's instructions. IL-1β, IL-6, and MCP-1 were measured in ELISAs from R&D Systems. IL-10 and IL-18 were measured in ELISAs from Invitrogen. Optical densities were measured using a SpectraMax Plus 354 plate reader (Molecular Devices) and concentrations were analyzed using a four-parameter curve fitting software (SoftMax 5.4.1).
Statistical Analyses
A two-tailed independent t-test was used to determine whether antibiotic affected numbers of colony forming units of commensal gut bacteria. Two-way repeated measures analyses of variance (ANOVA) were used to test for differences body weight between all groups of rats. Two-way ANOVAs were run to analyze the effect of stress or antibiotic on individual cytokines and chemokines. Data points were treated as outliers if they failed Grubb's test for outliers [53] and were also recorded as affected by experimental procedures by the experimenter. Data are presented as means ± the standard error of the mean. P<0.05 was considered statistically significant.
Results
Single exposure to tail shock activated the stress response
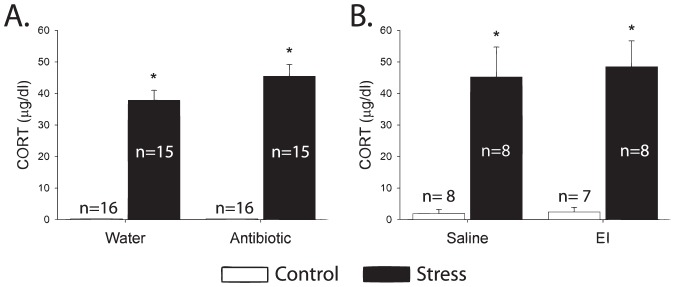
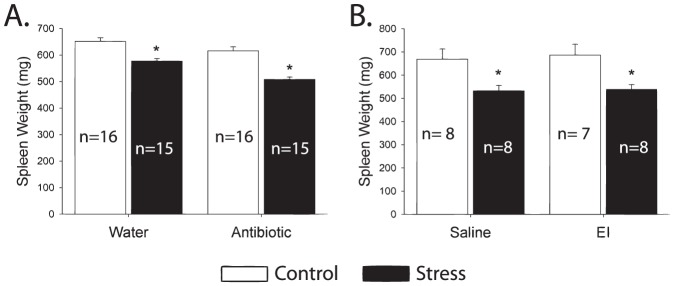
Consistent with prior work using this stressor [54]–[56], exposure to acute tail shock stress resulted in increased plasma corticosterone over control levels (p<0.001) ( Figure 1 ). Reduced spleen weight, indicating sympathetic nervous system activity [57], [58], was observed following stress independent of antibiotic or EI treatment (p<0.001) ( Figure 2 ). These values represent large changes from baseline and are indicative of a severe acute stressor.
Figure 1. Exposure to an acute stressor significantly increases circulating corticosterone.
The increase in circulating corticosterone is typical of acute activation of the hypothalamic-pituitary-adrenal axis such as that which occurs as part of activation of the stress response. Neither antibiotics (A) nor endotoxin inhibitor (B) impacted the corticosterone response to tail shock. (*p<0.05).
Figure 2. Exposure to an acute stressor causes significant splenic atrophy.
This increase is not impacted by either antibiotics (A) or endotoxin inhibitor (B). Splenic atrophy is typical of acute activation of the sympathetic nervous system such as that which occurs as part of activation of the stress response. (*p<0.05).
Antibiotic administration effectively reduced commensal bacterial load
The number of colony forming units measured in the fecal samples of rats receiving antibiotics was significantly lower than control rats (p<0.001) ( Figure 3A ). In fact, in all but one rat, the number of CFU observed in rats receiving antibiotics was below the detectable limit. This decrease in CFU count suggests that antibiotic administration significantly reduced the commensal bacteria, as expected. No decrease in body weight was observed, suggesting that antibiotics did not create other gross physiological changes that could confound the interpretation of the results ( Figure 3B ).
Figure 3. The efficacy of antibiotic administration is shown by successful reduction of colony forming units of bacteria in the absence of gross physiological changes such as a reduction in body weight gain.
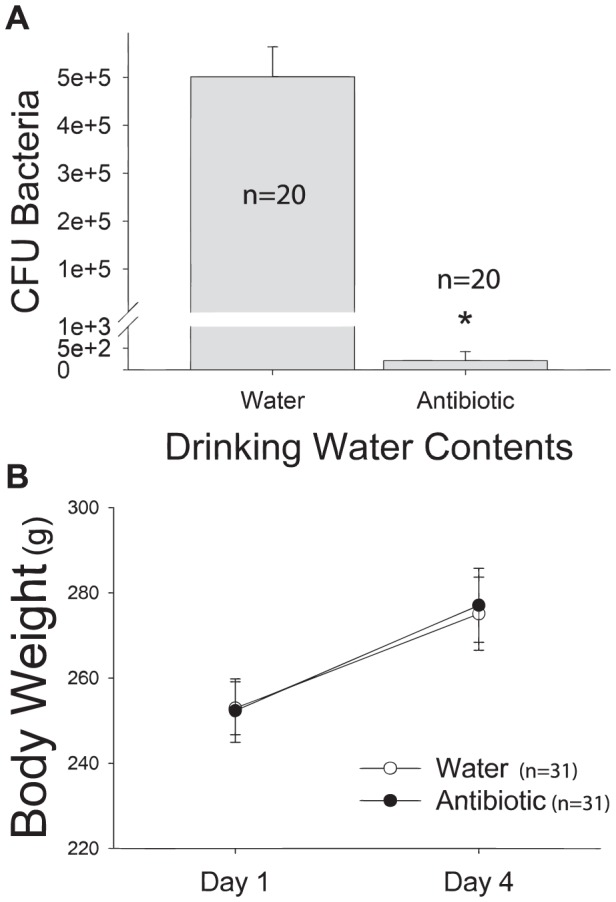
Figure 3A shows colony forming units of bacteria cultured on nutrient agar were significantly reduced in rats receiving antibiotic treatment. Although many species of commensal bacteria cannot be cultured on nutrient agar, the reduction produced by antibiotics is indicative of successful depletion of commensal bacteria by antibiotics, as neither the agar or the antibiotic regimen are specific for any particular group of bacteria. (*p<0.05). Figure 3B : depicts body weight changes across antibiotic treatment regimen. Body weights increased in all groups across time and were unaffected by antibiotic treatment. (*p<0.05).
Stress increased circulating LPS
Levels of LPS in the circulation are quite low at baseline reflecting adequate barrier function of the mucosal surfaces that contain the commensal flora. Exposure to acute stress increases the concentration of LPS measured in plasma (p<0.01) ( Figure 4 ). Levels of LPS measured in the circulation changed from 0.2708±0.0361 EU to 0.7084±0.1700 EU possibly reflecting changes to the mucosal environment in response to stress.
Figure 4. Stress evokes a significant increase in circulating concentrations of LPS.
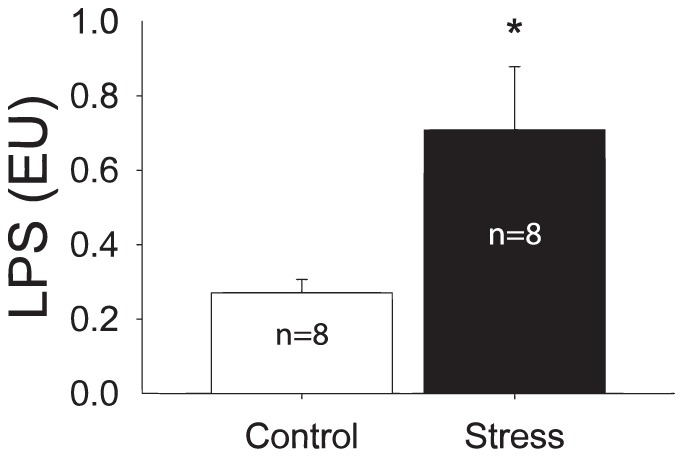
The systemic or circulating increased concentration of LPS is indicative of leakage of the commensal bacteria and their byproducts. (*p<0.05).
Acute-stress alters the relative abundance of Prevotella but does not impact overall diversity
16S rRNA analysis can reveal the relative abundance of all genera in the microbiome (including genus-level clusters of DNA sequences that have not yet been formally described). Stress caused a decrease in the relative abundance of a single genus, Prevotella. The decrease was detectable immediately following stress (p<0.05), and persisted for 24 hours after stressor termination (p<0.01), in fecal samples taken from the colon ( Figure 5a ). The relative abundance of Prevotella also decreased in cecal samples (p<0.01), although this decrease was only statistically significant 24 hrs after stressor termination ( Figure 5b ). There was no change α-diversity (the mean species diversity on a local scale, such as within a fecal sample) created by acute tail shock stress ( Figure 5c ). Similarly, stress did not impact β-diversity (differentiation in mean species diversity between collection sites).
Figure 5. The impact of stressor exposure on the commensal flora.
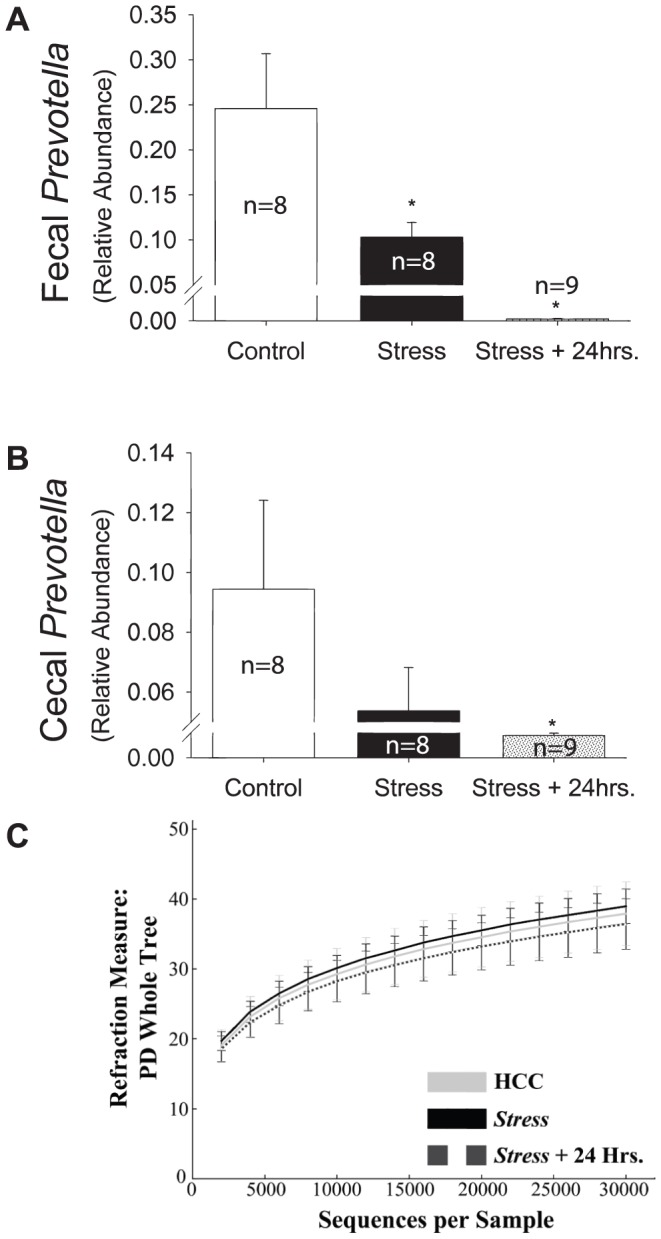
Figure 5A depicts the relative abundance of Prevotella decreased in fecal samples immediately following termination of the stressor. These changes persisted for at least 24 hours after the rats were returned to their home cages. (*p<0.05). Figure 5B shows the relative abundance of Prevotella decreased in cecal content samples. Although there was an immediate trend following the termination of the stressor, the difference in the relative abundance of Prevotella was only significant 24 hours later (*p<0.05). Figure 5C reveals no effect of stress on α-diversity in fecal samples. Cecal samples similarly showed no changes in overall diversity attributable to stress (data not shown).
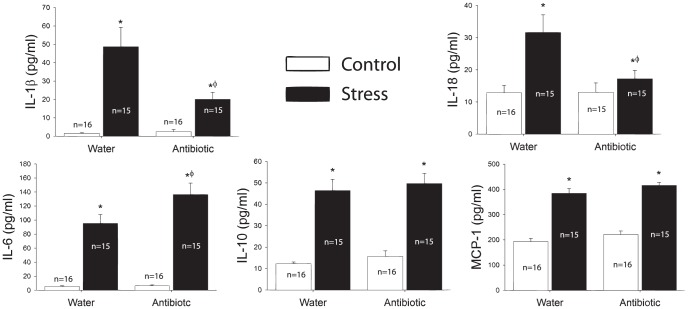
Antibiotic administration attenuated the production of some cytokines
Stress increased circulating levels of IL-1β (p<0.001), IL-6 (p<0.001), IL-10 (p<0.001), IL-18 (p<0.001), and MCP-1 (p<0.001) ( Figure 6 ). Administration of antibiotics attenuated the stress-induced production of IL-1β (p<0.01) and IL-18 (p<0.05) ( Figure 6 ). However, administration of antibiotics failed to attenuate the stress-induced production of IL-6, IL-10, and MCP-1 ( Figure 6 ). Interestingly, although antibiotics reduced the impact of stress on some cytokines, they increased circulating levels of IL-6 following stress (p<0.05).
Figure 6. Stress evokes a significant increase in circulating IL-1β, IL-6, IL-10, IL-18, and MCP-1.
Administration of antibiotics significantly attenuated the impact of stress on IL-1β and IL-18. Antibiotics, however, did not attenuate IL-10 or MCP-1, and actually increased levels of circulating IL-6. (*p<0.05 vs. control, water; Ф p<0.05 vs. stress, water).
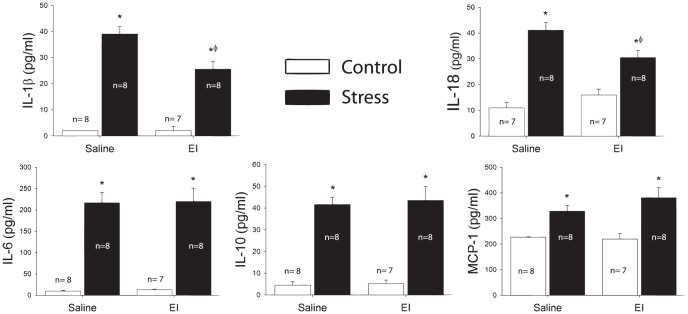
EI administration attenuated the production of the same cytokines as antibiotics
Stress again increased circulating levels of IL-1β (p<0.001), IL-6 (p<0.001), IL-10 (p<0.001, IL-18 (p<0.001), and MCP-1 (p<0.001) ( Figure 7 ). As with antibiotic administration, administration of EI attenuated the stress induced production of IL-1β (p<0.01) and IL-18 (p<0.05) ( Figure 7 ). Administration of EI also failed to attenuate the stress-induced production of IL-6, IL-10, and MCP-1 ( Figure 7 ).
Figure 7. Stress evokes a significant increase in circulating IL-1β, IL-6, IL-10, IL-18, and MCP-1.
Administration of endotoxin inhibitor significantly attenuated the impact of stress on IL-1β and IL-18. Endotoxin inhibitor, however, did not attenuate IL-6, IL-10, or MCP-1. (*p<0.05 vs. control, water; Ф p<0.05 vs. stress, water).
Discussion
The results support the hypothesis that commensal bacteria and LPS release contribute to stress-evoked increase in cytokines and chemokines in the blood. Adult male rats exposed to acute tail shock displayed robust elevations in plasma concentrations of several cytokines and chemokines including IL-1β, IL-6, IL-10, IL-18, and MCP-1. The administration of oral antibiotics or endotoxin inhibitor reduced the stress-induced elevation of IL-1β and IL-18, but interestingly, not IL-6, IL-10, or MCP-1. Thus, another signal beyond the commensal bacteria or released LPS is sufficient for synthesis and release of cytokines and chemokines such as IL-6, IL-10, and MCP-1 following stressor exposure.
Chronic stressors can create shifts in commensal bacterial diversity [9], and this change can impact peripheral immunity, as well as render the intestine vulnerable to pathogenic bacteria infection. Our results suggest that although intact commensal bacteria are necessary for stress-evoked increases in IL-1β and IL-18, these increases are not correlated with decreases in overall bacterial community diversity. Exposure to acute tail shock did not impact overall diversity (either α-diversity or β-diversity) in either fecal or cecal samples. Tail shock did, however, reduce the relative abundance of Prevotella. Prevotella is a highly prevalent genus of Gram-negative bacteria in the normal commensal bacterial community and has been reported to drive the overall composition of the flora [59]. Changes in the relative abundance of this genus may have broad physiological and immunological consequences. Reduced abundance of Prevotella can impact immunological inflammatory disease states such as inflammatory bowel disease [60], eczema [61], and rheumatoid arthritis patients [62]. Thus the stress-induced reduction in Prevotella may reduce anti-inflammatory status of the mucosal immune system and potentially contribute to the pro-inflammatory state produced by stress. Alternatively, the stress-induced reduction in Prevotella may result from death of these bacteria and which would result in the release of pro-inflammatory MAMPs, such as LPS as observed following stressor exposure.
Another goal of the current studies was to determine the specific role of LPS as a signaling molecule important for communication between commensal bacteria and the immune system. The current data support a signaling pathway involving LPS, released from the commensal bacteria in stress-induced cytokine and chemokine responses. Because stress increased circulating concentrations of LPS, leakage of LPS from the commensal bacteria may be important in stress-induced cytokine and chemokine production. Inhibiting LPS by administering EI produced the same effects as antibiotic administration, reducing stress-induced increases in IL-1β and IL-18 but not attenuating IL-6, IL-10, or MCP-1. Although the magnitude of the EI induced attenuation of stress induced IL-1β and IL-18 production was smaller than that observed with antibiotics, EI only inhibits the signaling action of LPS, whereas antibiotic treatment should also reduce other MAMPs such as peptidoglycans [63]. As in the antibiotic study, another signal was sufficient for stress-induced synthesis and release of IL-6, IL-10, and MCP-1.
The present study goes beyond prior work in several important respects. First, it examined a greater number of cytokines and chemokines than previous investigations, providing a broader view of the immune response. Second, it demonstrated that the commensal bacteria have a role in stress-induced inflammatory protein production following a single exposure to an acute stressor. Third, it described no effect of acute stressor exposure on intestinal diversity and a selective reduction in Prevotella. And finally, for the first time, it directly examined the mechanism by which the commensal bacteria (via LPS release) influence stress-induced cytokine and chemokine production. Our results thus reveal a novel role for the gut commensal bacteria in selective modulation of inflammatory proteins. This selectivity of the flora to impact IL-1β and IL-18 may help to fully reveal the mechanisms by which stress and the commensal flora impact immune function.
Recent studies highlight several features unique to the synthesis and release of IL-1β and IL-18 that are not necessary for the production of other cytokines and chemokines such as IL-6, IL-10, or MCP-1 [64]. The unique features in the synthesis pathways of these proteins result in different signaling requirements for these two families of inflammatory proteins [65] and could, thus, explain the selectivity of the antibiotic or EI induced attenuation in the stress-induced cytokine and chemokine response. Of particular importance, while IL-6, IL-10, MCP-1, and the majority of other cytokines and chemokines are synthesized in their releasable form, IL-1β and IL-18 are synthesized as inactive precursors [64], [66]. Processing, therefore, is required in the complete synthesis and release pathway for IL-1β and IL-18. This post-translational processing is predominately mediated by caspase-1, an enzyme activated upon the assembly of a multimeric signaling complex called the inflammasome [67]–[69]. Recent data suggests that the inflammasome is involved in stress-evoked cytokine and chemokine production [70]. Given that antibiotics and EI selectively affect IL-1β and IL-18, these data suggest an interaction between the stress response, the commensal bacteria, and the inflammasome in stress-induced cytokine and chemokine production.
Investigations examining the inflammasome have highlighted the necessity of two signals for inflammasome assembly or activation. The first signal leads to synthesis of components of the inflammasome, as well as pro-IL-1β, and pro-IL-18 [71], [72]. Inflammasome independent cytokines are also completely synthesized in response to a single signal [73]. The second signal leads to final inflammasome assembly and caspase-1 activation [67], [74]. In vitro, the requirement for two signals has been demonstrated using a MAMP and a danger associated molecular pattern (DAMP) such as ATP, Hsp72, Uric Acid, or even elevated concentrations of glucose [65], [71], [75]. Administration of a MAMP [65], [76], [77] or DAMP [78] alone is not capable of activating the inflammasome, however, co-administration of both ligands is sufficient for inflammasome and cytokine production.
Although the exact nature of the requirement for the combination MAMPs and DAMPs is unknown, neutralizing only a single MAMP was sufficient to selectively suppress the inflammasome dependent cytokines in vivo. Because MAMPs from the commensal bacteria were only necessary for stress-induced synthesis of inflammasome dependent cytokines, MAMPs likely provide the second signal necessary for inflammasome activation. Although speculative, it seems probable that in vivo after exposure to an acute stressor, DAMPs likely act as the first signal in stress-evoked cytokine and chemokine production. DAMPs such as Hsp72 and uric acid are known to increase in response to many stressors [79]–[81] including tail shock [55], [56], [70], [82]–[84]. Furthermore, DAMPs, as well as stress-evoked IL-6, IL-10, or MCP-1 responses are not impacted by either antibiotic or EI treatment. Thus, DAMPs may underlie the stress-induced release of the inflammasome independent cytokines and chemokines and hence, act as the first signal in the inflammasomal pathway of stress-evoked cytokine and chemokine production. Other secretions of the stress response such as catecholamines may also act as the first signal in stress-evoked cytokine and chemokine production [85], [86].
The present study is the first to demonstrate that release of stress-inducible inflammasome dependent inflammatory proteins depend on commensal bacteria. It is also the first to establish that commensal bacteria mediate acute stress-induced immune activity, and to directly demonstrate a role for MAMPs in this process. Each of these findings provides a novel mechanistic description of how exposure to stressors elevates blood concentrations of cytokines and chemokines. Furthermore, the aggregate of these findings alludes to a novel, inflammasomal pathway for stress-induced cytokine and chemokine production as summarized in Figure 8 . Further examination of the interplay between the commensal bacteria and the inflammasome is important and may result in the development of therapeutic candidates that can suppress the cytokine storm evoked by severe stressors or trauma [70], [87], [88].
Figure 8. Exposure to a stressor activates the hypothalamic-pituitary-adrenal axis and sympathetic nervous system resulting in changes to the commensal flora (including a decrease in prevotella) and the release of microbe associated molecular patterns (MAMPs) as well as the the release of danger associated molecular patterns (DAMPs) either actively or via cell death.
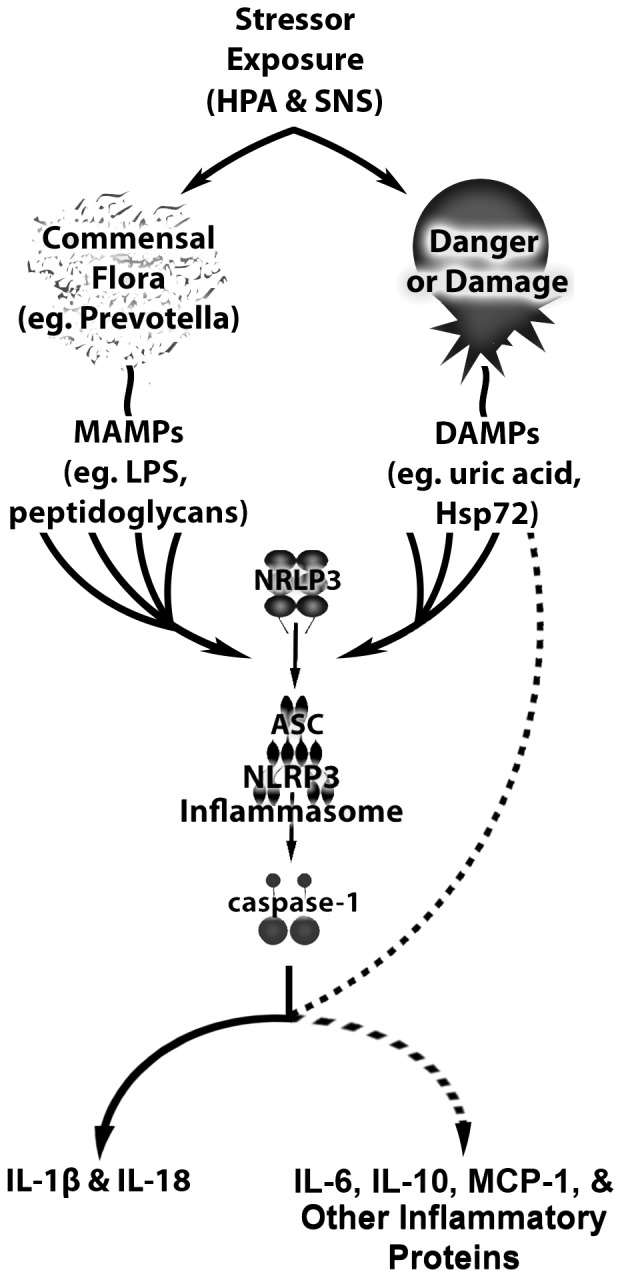
DAMP and MAMP signals then converge upon the inflammasome to yield IL-1β and IL-18 production. DAMPs may also act to drive responses from inflammasome independent inflammatory proteins including IL-6, IL-10, and MCP-1.
Funding Statement
The studies published in this manuscript were funded by the National Science Foundation, grant number IOS 1022451. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Bailey MT, Dowd SE, Galley JD, Hufnagle AR, Allen RG, et al. (2011) Exposure to a social stressor alters the structure of the intestinal microbiota: Implications for stressor-induced immunomodulation. Brain Behav Immun 25: 397–407 Available: http://www.ncbi.nlm.nih.gov/pubmed/21040780?dopt=Citation. Accessed 2012 Nov 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Macpherson AJ, Uhr T (2004) Compartmentalization of the mucosal immune responses to commensal intestinal bacteria. Annals of the New York Academy of Sciences 1029: 36–43 Available: http://www.ncbi.nlm.nih.gov/pubmed/15681741. Accessed 2012 March 21. [DOI] [PubMed] [Google Scholar]
- 3. Shanahan F (2002) The host-microbe interface within the gut. Best practice & research Clinical gastroenterology 16: 915–931 Available: http://www.ncbi.nlm.nih.gov/pubmed/12473298. Accessed 2012 March 22 March. [DOI] [PubMed] [Google Scholar]
- 4. Chung H, Pamp SJ, Hill JA, Surana NK, Edelman SM, et al. (2012) Gut Immune Maturation Depends on Colonization with a Host-Specific Microbiota. Cell 149: 1578–1593 Available: http://dx.doi.org/10.1016/j.cell.2012.04.037. Accessed 2012 June 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Keita AV, Söderholm JD (2010) The intestinal barrier and its regulation by neuroimmune factors. Neurogastroenterology and motility: the official journal of the European Gastrointestinal Motility Society 22: 718–733 Available: http://www.ncbi.nlm.nih.gov/pubmed/20377785. Accessed 2011 Oct 28. [DOI] [PubMed] [Google Scholar]
- 6. Tlaskalová-Hogenová H, Stepánková R, Hudcovic T, Tucková L, Cukrowska B, et al. (2004) Commensal bacteria (normal microflora), mucosal immunity and chronic inflammatory and autoimmune diseases. Immunology letters 93: 97–108 Available: http://www.ncbi.nlm.nih.gov/pubmed/15158604. Accessed 2011 June 14. [DOI] [PubMed] [Google Scholar]
- 7. Kranich J, Maslowski KM, Mackay CR (2011) Commensal flora and the regulation of inflammatory and autoimmune responses. Seminars in immunology 23: 139–145 Available: http://dx.doi.org/10.1016/j.smim.2011.01.011. Accessed 2012 March 9. [DOI] [PubMed] [Google Scholar]
- 8. Turnbaugh PJ, Ridaura VK, Faith JJ, Rey FE, Knight R, et al. (2009) The effect of diet on the human gut microbiome: a metagenomic analysis in humanized gnotobiotic mice. Science translational medicine 1: 6ra14 Available: http://stm.sciencemag.org/content/1/6/6ra14.abstract. Accessed 2012 March 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bailey MT, Dowd SE, Parry NMA, Galley JD, Schauer DB, et al. (2010) Stressor exposure disrupts commensal microbial populations in the intestines and leads to increased colonization by Citrobacter rodentium. Infection and immunity 78: 1509–1519 Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2849416&tool=pmcentrez&rendertype=abstract. Accessed 2012 March 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yang PC, Jury J, Soderholm JD, Sherman PM, McKay DM, et al. (2006) Chronic psychological stress in rats induces intestinal sensitization to luminal antigens. Am J Pathol 168: 104–114 Available:http://www.ncbi.nlm.nih.gov/pubmed/16400013?dopt=Citation. Accessed 2012 Nov 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bailey MT, Lubach GR, Coe CL (2004) Prenatal stress alters bacterial colonization of the gut in infant monkeys. Journal of pediatric gastroenterology and nutrition 38: 414–421 Available:http://www.ncbi.nlm.nih.gov/pubmed/15085020. Accessed 2012 March 21. [DOI] [PubMed] [Google Scholar]
- 12. Liu T, Wang BQ, Wang CS, Yang PC (2006) Concurrent exposure to thermal stress and oral Ag induces intestinal sensitization in the mouse by a mechanism of regulation of IL-12 expression. 84: 430–439 Available: http://dx.doi.org/10.1111/j.1440-1711.2006.01452.x. Accessed 2012 Nov 9. [DOI] [PubMed] [Google Scholar]
- 13. Bailey MT, Engler H, Sheridan JF (2006) Stress induces the translocation of cutaneous and gastrointestinal microflora to secondary lymphoid organs of C57BL/6 mice. J Neuroimmunol 171: 29–37 Available: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16253348. Accessed 2012 Nov 9. [DOI] [PubMed] [Google Scholar]
- 14. Santos J, Benjamin M, Yang PC, Prior T, Perdue MH (2000) Chronic stress impairs rat growth and jejunal epithelial barrier function: role of mast cells. Am J Physiol Gastrointest Liver Physiol 278: G847–54 Available: http://www.ncbi.nlm.nih.gov/pubmed/10859213?dopt=Citation. Accessed 2012 Nov 9. [DOI] [PubMed] [Google Scholar]
- 15. Lundgren O (2000) Sympathetic input into the enteric nervous system. Gut 47: 33iv–35 Available: http://gut.bmj.com. Accessed 2012 April 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Knowles SR, Nelson EA, Palombo EA (2008) Investigating the role of perceived stress on bacterial flora activity and salivary cortisol secretion: a possible mechanism underlying susceptibility to illness. Biological psychology 77: 132–137 Available: http://www.ncbi.nlm.nih.gov/pubmed/18023961. Accessed 2012 March 9. [DOI] [PubMed] [Google Scholar]
- 17. Bailey MT, Coe CL (1999) Maternal separation disrupts the integrity of the intestinal microflora in infant rhesus monkeys. Developmental psychobiology 35: 146–155 Available: http://www.ncbi.nlm.nih.gov/pubmed/10461128. Accessed 2012 March 21. [PubMed] [Google Scholar]
- 18. Allen RG, Lafuse WP, Galley JD, Ali MM, Ahmer BMM, et al. (2012) The intestinal microbiota are necessary for stressor-induced enhancement of splenic macrophage microbicidal activity. Brain Behav Immun 26: 382–371 Available: http://dx.doi.org/10.1016/j.bbi.2011.11.002. Accessed 2012 March 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fleshner M, Johnson JD, Friedman J (2007) Extracellular Hsp 72: A Double-Edged Sword for Host Defense. Heat Shock Proteins: Potent Mediators of Inflammation and Immunity 235–263 Available: http://dx.doi.org/10.1007/978-1-4020-5585-0_15. Accessed 2012 Nov 9.
- 20. Lambert GP (2009) Stress-induced gastrointestinal barrier dysfunction and its inflammatory effects. Journal of animal science 87: E101–8 Available: http://jas.fass.org/cgi/content/abstract/87/14_suppl/E101. Accessed 2012 March 4. [DOI] [PubMed] [Google Scholar]
- 21. You Z, Luo C, Zhang W, Chen Y, He J, et al. (2011) Pro- and anti-inflammatory cytokines expression in rat's brain and spleen exposed to chronic mild stress: Involvement in depression. Behavioural brain research 225: 135–141 Available: http://www.ncbi.nlm.nih.gov/pubmed/21767575. Accessed 2011 July 22. [DOI] [PubMed] [Google Scholar]
- 22. Bailey MT, Kinsey SG, Padgett DA, Sheridan JF (2009) Social stress enhances IL-1 and TNF- production by Porphyromonas gingivalis lipopolysaccharide-stimulated CD11b+ cells. Physiol Behav 98: 351–358 Available: http://www.ncbi.nlm.nih.gov/pubmed/19560480?dopt=Citation. Accessed 2012 Nov 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Johnson JD, Cortez V, Kennedy SL, Foley TE, Hanson H 3rd, et al. (2008) Role of central beta-adrenergic receptors in regulating proinflammatory cytokine responses to a peripheral bacterial challenge. Brain Behav Immun 22: 1078–1086 Available: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=18468841. Accessed 2012 Nov 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Maslanik T, Bernstein-Hanley I, Helwig B, Fleshner M (2012) The impact of acute-stressor exposure on splenic innate immunity: A gene expression analysis. Brain Behav Immun 26: 142–149 Available: http://www.ncbi.nlm.nih.gov/pubmed/21893187. Accessed 2011 Nov 11. [DOI] [PubMed] [Google Scholar]
- 25. Barker LA, Dazin PF, Levine JD, Green PG (2005) Sympathoadrenal-dependent sexually dimorphic effect of nonhabituating stress on in vivo neutrophil recruitment in the rat. British journal of pharmacology 145: 872–879 Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1576213&tool=pmcentrez&rendertype=abstract. Accessed 2011 Oct 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Girotti M, Donegan JJ, Morilak DA (2011) Chronic intermittent cold stress sensitizes neuro-immune reactivity in the rat brain. Psychoneuroendocrinology 36: 1164–1174 Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3130087&tool=pmcentrez&rendertype=abstract. Accessed 2011 Nov 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Guéguinou N, Bojados M, Jamon M, Derradji H, Baatout S, et al. (2011) Stress response and humoral immune system alterations related to chronic hypergravity in mice. Psychoneuroendocrinology Available: http://www.ncbi.nlm.nih.gov/pubmed/21724335. Accessed 2011 Nov 17. [DOI] [PubMed]
- 28. Ricart-Jané D, Rodríguez-Sureda V, Benavides A, Peinado-Onsurbe J, López-Tejero MD, et al. (2002) Immobilization stress alters intermediate metabolism and circulating lipoproteins in the rat. Metabolism: clinical and experimental 51: 925–931 Available: http://www.ncbi.nlm.nih.gov/pubmed/12077743. Accessed 2012 June 14. [DOI] [PubMed] [Google Scholar]
- 29. Martí O, Martí J, Armario A (1994) Effects of chronic stress on food intake in rats: influence of stressor intensity and duration of daily exposure. Physiology & behavior 55: 747–753 Available: http://www.ncbi.nlm.nih.gov/pubmed/8190805. Accessed 2012 April 5. [DOI] [PubMed] [Google Scholar]
- 30. Kalueff AV, Tuohimaa P (2004) Grooming analysis algorithm for neurobehavioural stress research. Brain research Brain research protocols 13: 151–158 Available: http://dx.doi.org/10.1016/j.brainresprot.2004.04.002. Accessed 2012 March 22. [DOI] [PubMed] [Google Scholar]
- 31. Campisi J, Leem TH, Fleshner M (2002) Acute stress decreases inflammation at the site of infection. A role for nitric oxide. Physiol Behav 77: 291–299 Available: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=12419405. Accessed 2012 Nov 9. [DOI] [PubMed] [Google Scholar]
- 32. Gazda LS, Smith T, Watkins LR, Maier SF, Fleshner M (2003) Stressor exposure produces long-term reductions in antigen-specific T and B cell responses. Stress 6: 259–267 Available: http://www.ncbi.nlm.nih.gov/pubmed/14660058?dopt=Citation. Accessed 2012 Nov 9. [DOI] [PubMed] [Google Scholar]
- 33. Campisi J, Leem TH, Fleshner M (2003) Stress-induced extracellular Hsp72 is a functionally significant danger signal to the immune system. Cell Stress Chaperones 8: 272–286 Available: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=14984061. Accessed 2012 Nov 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Campisi J, Sharkey C, Johnson JD, Asea A, Maslanik T, et al. (2012) Stress-induced facilitation of host response to bacterial challenge in F344 rats is dependent on extracellular heat shock protein 72 and independent of alpha beta T cells. Stress Available: http://www.ncbi.nlm.nih.gov/pubmed/22217161. Accessed 2012 June 11. [DOI] [PubMed]
- 35. Campisi J, Fleshner M (2003) Role of extracellular HSP72 in acute stress-induced potentiation of innate immunity in active rats. J Appl Physiol 94: 43–52 Available: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=12391077. Accessed 2012 Nov 9. [DOI] [PubMed] [Google Scholar]
- 36. Fleshner M, Hermann J, Lockwood LL, Laudenslager ML, Watkins LR, et al. (1995) Stressed rats fail to expand the CD45RC+CD4+ (Th1-like) T cell subset in response to KLH: possible involvement of IFN-gamma. Brain Behav Immun 9: 101–112 Available: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=7549034. Accessed 2012 Nov 9. [DOI] [PubMed] [Google Scholar]
- 37. Moraska A, Campisi J, Nguyen KT, Maier SF, Watkins LR, et al. (2002) Elevated IL-1beta contributes to antibody suppression produced by stress. J Appl Physiol 93: 207–215 Available: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=12070207. Accessed 2012 Nov 9. [DOI] [PubMed] [Google Scholar]
- 38. Dhabhar FS (2000) Acute Stress Enhances While Chronic Stress Suppresses Skin Immunity: The Role of Stress Hormones and Leukocyte Trafficking. Annals of the New York Academy of Sciences 917: 876–893 Available: http://dx.doi.org/10.1111/j.1749-6632.2000.tb05454.x. Accessed 2012 Nov 9. [DOI] [PubMed] [Google Scholar]
- 39. Maier SF, Watkins LR, Fleshner M (1994) Psychoneuroimmunology. The interface between behavior, brain, and immunity. The American psychologist 49: 1004–1017 Available: http://www.ncbi.nlm.nih.gov/pubmed/7818221. Accessed 2012 Sept 10. [DOI] [PubMed] [Google Scholar]
- 40. Sudo N, Chida Y, Aiba Y, Sonoda J, Oyama N, et al. (2004) Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. The Journal of physiology 558: 263–275 Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1664925&tool=pmcentrez&rendertype=abstract. Accessed 2012 July 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sambol JT, Forsythe RM, Deitch EA (2001) Gut Barrier Failure. In: Souba WW, Wilmore DV, editors. Surgical Research. Academic Press. pp. 599–611. Available: http://books.google.com/books?id=OyQnRD5LjnAC&pgis=1. Accessed 2012 Jan 23.
- 42. Caso JR, Hurtado O, Pereira MP, Garcia-Bueno B, Menchen L, et al. (2009) Colonic bacterial translocation as a possible factor in stress-worsening experimental stroke outcome 10.1152/ajpregu.90825.2008. Am J Physiol Regul Integr Comp Physiol 296: R979–985 Available: http://ajpregu.physiology.org/cgi/content/abstract/296/4/R979. Accessed 2012 Nov 9. [DOI] [PubMed] [Google Scholar]
- 43. Hoggatt AF, Hoggatt J, Honerlaw M, Pelus LM (2010) A spoonful of sugar helps the medicine go down: a novel technique to improve oral gavage in mice. J Am Assoc Lab Anim Sci 49: 329–334 Available: http://www.ncbi.nlm.nih.gov/pubmed/20587165?dopt=Citation. Accessed 2012 Nov 9. [PMC free article] [PubMed] [Google Scholar]
- 44. Brown AP, Dinger N, Levine BS (2000) Stress produced by gavage administration in the rat. Contemporary topics in laboratory animal science/American Association for Laboratory Animal Science. 39: 17–21 Available: http://www.ncbi.nlm.nih.gov/pubmed/11178310. Accessed 2012 Jan 23. [PubMed] [Google Scholar]
- 45. Katafuchi T (2003) Endotoxin inhibitor blocks heat exposure-induced expression of brain cytokine mRNA in aged rats. Molecular Brain Research 118: 24–32 Available: http://dx.doi.org/10.1016/S0169-328X(03)00331-0. Accessed 2012 Jan 3. [DOI] [PubMed] [Google Scholar]
- 46. Kuhn R, Schubert D, Tautenhahn J, Nestler G, Schulz HU, et al. (2005) Effect of intraperitoneal application of an endotoxin inhibitor on survival time in a laparoscopic model of peritonitis in rats. World journal of surgery 29: 766–770 Available: http://www.springerlink.com/content/k58mh41w4656060w/. Accessed 2011 Oct 6. [DOI] [PubMed] [Google Scholar]
- 47. Costello EK, Lauber CL, Hamady M, Fierer N, Gordon JI, et al. (2009) Bacterial community variation in human body habitats across space and time. Science (New York, NY) 326: 1694–1697 Available: http://www.sciencemag.org/content/326/5960/1694.abstract. Accessed 2012 Feb 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Caporaso JG, Lauber CL, Walters WA, Berg-Lyons D, Lozupone CA, et al. (2011) Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proceedings of the National Academy of Sciences of the United States of America 108 Suppl: 4516–4522 Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3063599&tool=pmcentrez&rendertype=abstract. Accessed 2011 July 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, et al. (2010) QIIME allows analysis of high-throughput community sequencing data. Nature methods 7: 335–336 Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3156573&tool=pmcentrez&rendertype=abstract. Accessed 2011 Aug 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Edgar RC (2010) Search and clustering orders of magnitude faster than BLAST. Bioinformatics (Oxford, England) 26: 2460–2461 Available: http://www.ncbi.nlm.nih.gov/pubmed/20709691. Accessed 2012 March 9. [DOI] [PubMed] [Google Scholar]
- 51. Cole JR, Wang Q, Cardenas E, Fish J, Chai B, et al. (2009) The Ribosomal Database Project: improved alignments and new tools for rRNA analysis. Nucleic acids research 37: D141–5 Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2686447&tool=pmcentrez&rendertype=abstract. Accessed 2011 July 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. DeSantis TZ, Hugenholtz P, Larsen N, Rojas M, Brodie EL, et al. (2006) Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Applied and environmental microbiology 72: 5069–5072 Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1489311&tool=pmcentrez&rendertype=abstract. Accessed 2011 June 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Grubbs FE (1969) Procedures for Detecting Outlying Observations in Samples. Technometrics 11: 1–21. [Google Scholar]
- 54. Fleshner M, Brennan FX, Nguyen K, Watkins LR, Maier SF (1996) RU-486 blocks differentially suppressive effect of stress on in vivo anti-KLH immunoglobulin response. Am J Physiol 271: R1344–52 Available: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=8945973. Accessed 2012 Nov 9. [DOI] [PubMed] [Google Scholar]
- 55. Nickerson M, Kennedy SL, Johnson JD, Fleshner M (2006) Sexual dimorphism of the intracellular heat shock protein 72 response 10.1152/japplphysiol.00259.2006. J Appl Physiol 101: 566–575 Available: http://jap.physiology.org/cgi/content/abstract/101/2/566. Accessed 2012 Nov 9. [DOI] [PubMed] [Google Scholar]
- 56. Campisi J, Leem TH, Greenwood BN, Hansen MK, Moraska A, et al. (2003) Habitual physical activity facilitates stress-induced HSP72 induction in brain, peripheral, and immune tissues. Am J Physiol Regul Integr Comp Physiol 284: R520–30 Available: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=12399251. Accessed 2012 Nov 9. [DOI] [PubMed] [Google Scholar]
- 57. Kuwahira I, Kamiya U, Iwamoto T, Moue Y, Urano T, et al. (1999) Splenic contraction-induced reversible increase in hemoglobin concentration in intermittent hypoxia. J Appl Physiol 86: 181–187 Available: http://jap.physiology.org/cgi/content/abstract/86/1/181. Accessed 2012 June 21. [DOI] [PubMed] [Google Scholar]
- 58. Hochachka PW, Liggins GC, Guyton GP, Schneider RC, Stanek KS, et al. (1995) Hormonal regulatory adjustments during voluntary diving in Weddell seals. Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology 112: 361–375 Available: http://dx.doi.org/10.1016/0305-0491(96)85239-4. Accessed 2012 June 21. [DOI] [PubMed] [Google Scholar]
- 59. Yatsunenko T, Rey FE, Manary MJ, Trehan I, Dominguez-Bello MG, et al. (2012) Human gut microbiome viewed across age and geography. Nature advance on Available: http://dx.doi.org/10.1038/nature11053. Accessed 2012 May 9. [DOI] [PMC free article] [PubMed]
- 60. Seksik P, Rigottier-Gois L, Gramet G, Sutren M, Pochart P, et al. (2003) Alterations of the dominant faecal bacterial groups in patients with Crohn's disease of the colon. Gut 52: 237–242 Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1774977&tool=pmcentrez&rendertype=abstract. Accessed 2012 Feb 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Mah KW, Björkstén B, Lee BW, van Bever HP, Shek LP, et al. (2006) Distinct pattern of commensal gut microbiota in toddlers with eczema. International archives of allergy and immunology 140: 157–163 Available: http://content.karger.com/ProdukteDB/produkte.asp?Aktion=ShowAbstract&ArtikelNr=92555&Ausgabe=231776&ProduktNr=224161. Accessed 2012 Jan 22 January. [DOI] [PubMed] [Google Scholar]
- 62. Vaahtovuo J, Munukka E, Korkeamäki M, Luukkainen R, Toivanen P (2008) Fecal microbiota in early rheumatoid arthritis. The Journal of rheumatology 35: 1500–1505 Available: http://www.ncbi.nlm.nih.gov/pubmed/18528968. Accessed 2011 Nov 17. [PubMed] [Google Scholar]
- 63. Mychajlonka M, McDowell TD, Shockman GD (1980) Inhibition of peptidoglycan, ribonucleic acid, and protein synthesis in tolerant strains of Streptococcus mutans. Antimicrobial agents and chemotherapy 17: 572–582 Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=283834&tool=pmcentrez&rendertype=abstract. Accessed 2012 June 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Eder C (2009) Mechanisms of interleukin-1beta release. Immunobiology 214: 543–553 Available: http://dx.doi.org/10.1016/j.imbio.2008.11.007. Accessed 2011 July 25. [DOI] [PubMed] [Google Scholar]
- 65. Netea MG, Simon A, van de Veerdonk F, Kullberg BJ, Van der Meer JWM, et al. (2010) IL-1beta processing in host defense: beyond the inflammasomes. PLoS pathogens 6: e1000661 Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2829053&tool=pmcentrez&rendertype=abstract. Accessed 2011 Aug 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Schroder JKT (2010) The inflammasomes. Cell 140: 821–832 Available: http://www.ncbi.nlm.nih.gov/pubmed?term=the inflammasomes kate schroder. Accessed 2012 Nov 9.20303873 [Google Scholar]
- 67. Tschopp J, Schroder K (2010) NLRP3 inflammasome activation: The convergence of multiple signalling pathways on ROS production? Nat Rev Immunol 10: 210–215 Available: http://dx.doi.org/10.1038/nri2725. Accessed 2011 June 28. [DOI] [PubMed] [Google Scholar]
- 68. Schroder K, Zhou R, Tschopp J (2010) The NLRP3 inflammasome: a sensor for metabolic danger? Science 327: 296–300 Available: http://www.ncbi.nlm.nih.gov/pubmed/20075245?dopt=Citation. Accessed 2012 Nov 9. [DOI] [PubMed] [Google Scholar]
- 69. Menu P, Vince JE (2011) The NLRP3 inflammasome in health and disease: the good, the bad and the ugly. Clin Exp Immunol 166: 1–15 Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3193914&tool=pmcentrez&rendertype=abstract. Accessed 2011 Sept 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Maslanik T, Mahaffey L, Tannura K, Beninson LA, Greenwood BN, et al. (2012) The inflammasome and Danger Associated Molecular Patterns (DAMPs) are implicated in cytokine and chemokine responses following stressor exposure. Brain Behav Immun In Press. [DOI] [PubMed] [Google Scholar]
- 71. Bauernfeind FG, Horvath G, Stutz A, Alnemri ES, MacDonald K, et al. (2009) Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J Immunol 183: 787–791 Available: http://www.jimmunol.org/cgi/content/abstract/183/2/787. Accessed 2011 June 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Masters SL, Dunne A, Subramanian SL, Hull RL, Tannahill GM, et al. (2010) Activation of the NLRP3 inflammasome by islet amyloid polypeptide provides a mechanism for enhanced IL-1β in type 2 diabetes. Nature immunology 11: 897–904 Available: http://dx.doi.org/10.1038/ni.1935. Accessed 2011 July 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Hamon Y, Luciani MF, Becq F, Verrier B, Rubartelli A, et al. (1997) Interleukin-1beta secretion is impaired by inhibitors of the Atp binding cassette transporter, ABC1. Blood 90: 2911–2915 Available: http://www.ncbi.nlm.nih.gov/pubmed/9376570. Accessed 2012 Sept 13. [PubMed] [Google Scholar]
- 74. Lamkanfi M, Mueller JL, Vitari AC, Misaghi S, Fedorova A, et al. (2009) Glyburide inhibits the Cryopyrin/Nalp3 inflammasome. The Journal of cell biology 187: 61–70 Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2762099&tool=pmcentrez&rendertype=abstract. Accessed 2011 Aug 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Kumar H, Kumagai Y, Tsuchida T, Koenig PA, Satoh T, et al. (2009) Involvement of the NLRP3 inflammasome in innate and humoral adaptive immune responses to fungal beta-glucan. J Immunol 183: 8061–8067 Available: http://www.jimmunol.org/cgi/content/abstract/183/12/8061. Accessed 2011 Oct 3. [DOI] [PubMed] [Google Scholar]
- 76. Griffiths RJ, Stam EJ, Downs JT, Otterness IG (1995) ATP induces the release of IL-1 from LPS-primed cells in vivo. Journal of immunology 154: 2821–2828 Available: http://www.ncbi.nlm.nih.gov/pubmed/7876552. Accessed 2011 Nov 15. [PubMed] [Google Scholar]
- 77. Piccini A, Carta S, Tassi S, Lasiglié D, Fossati G, et al. (2008) ATP is released by monocytes stimulated with pathogen-sensing receptor ligands and induces IL-1beta and IL-18 secretion in an autocrine way. Proceedings of the National Academy of Sciences of the United States of America 105: 8067–8072 Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2430360&tool=pmcentrez&rendertype=abstract. Accessed 2011 Sept 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Eisenbarth SC, Colegio OR, OConnor W, Sutterwala FS, Flavell RA (2008) Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature 453: 1122–1126 Available: http://dx.doi.org/10.1038/nature06939. Accessed 2012 Nov 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Walsh RC, Koukoulas I, Garnham A, Moseley PL, Hargreaves M, et al. (2001) Exercise increases serum Hsp72 in humans. Cell Stress Chaperones 6: 386–393 Available: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=11795476. Accessed 2012 Nov 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Febbraio MA, Ott P, Nielsen HB, Steensberg A, Keller C, et al. (2002) Exercise induces hepatosplanchnic release of heat shock protein 72 in humans. J Physiol 544: 957–962 Available: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=12411538. Accessed 2012 Nov 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Fleshner M, Campisi J, Amiri L, Diamond DM (2004) Cat exposure induces both intra- and extracellular Hsp72: the role of adrenal hormones. Psychoneuroendocrinology 29: 1142–1152 Available: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15219638. Accessed 2012 Nov 9. [DOI] [PubMed] [Google Scholar]
- 82. Fleshner M, Johnson JD (2005) Endogenous extra-cellular heat shock protein 72: releasing signal(s) and function. Int J Hyperthermia 21: 457–471 Available: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16048842. Accessed 2012 Nov 9. [DOI] [PubMed] [Google Scholar]
- 83. Johnson JD, Fleshner M (2006) Releasing signals, secretory pathways, and immune function of endogenous extracellular heat shock protein 72. J Leukoc Biol 79: 425–434 Available: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16387837. Accessed 2012 Nov 9. [DOI] [PubMed] [Google Scholar]
- 84.Fleshner M, Maslanik T, Beninson LA (2010) In Vivo Tissue Source and Releasing Signal for Endogenous Extracellular Hsp72. In: Asea A, Pedersen BK, editors. Heat Shock Proteins and Whole Body Physiology. Vol. 5. pp. 193–215.
- 85. Johnson JD, Campisi J, Sharkey CM, Kennedy SL, Nickerson M, et al. (2005) Catecholamines mediate stress-induced increases in peripheral and central inflammatory cytokines. Neuroscience 135: 1295–1307 Available: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16165282. Accessed 2012 Nov 9. [DOI] [PubMed] [Google Scholar]
- 86. Grisanti LA, Woster AP, Dahlman J, Sauter ER, Combs CK, et al. (2011) α1-adrenergic receptors positively regulate Toll-like receptor cytokine production from human monocytes and macrophages. The Journal of pharmacology and experimental therapeutics 338: 648–657 Available: http://jpet.aspetjournals.org/cgi/content/abstract/jpet.110.178012v1. Accessed Accessed 2012 Nov 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Frink M, Lu A, Thobe BM, Hsieh YC, Choudhry MA, et al. (2007) Monocyte chemoattractant protein-1 influences trauma-hemorrhage-induced distal organ damage via regulation of keratinocyte-derived chemokine production. Am J Physiol: Regul, Integr Comp Physiol 292: R1110–6 Available: http://ajpregu.physiology.org/cgi/content/abstract/292/3/R1110. Accessed 2012 May 14. [DOI] [PubMed] [Google Scholar]
- 88. Namas R, Ghuma A, Hermus L, Zamora R, Okonkwo DO, et al. (2009) The acute inflammatory response in trauma/hemorrhage and traumatic brain injury: current state and emerging prospects. The Libyan journal of medicine 4: 97–103 Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3066737&tool=pmcentrez&rendertype=abstract. Accessed 2012 March 19. [DOI] [PMC free article] [PubMed] [Google Scholar]