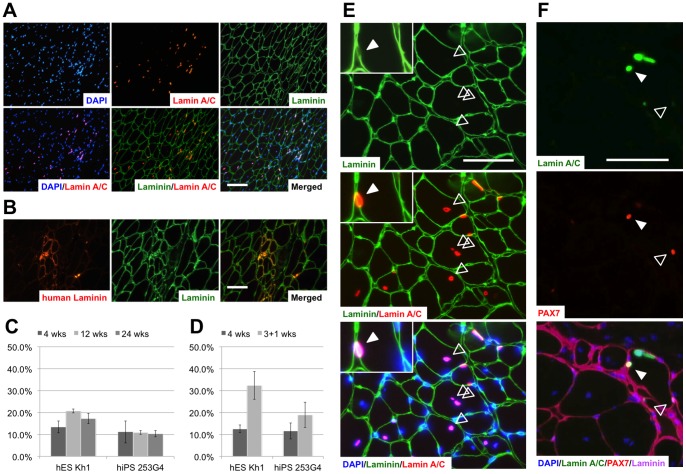
Figure 4. Engraftment of myogenic progenitors in damaged muscles of immunodeficient mice.
(A) Human nuclei labeled with human-specific lamin A/C localized mainly inside muscle fibers surrounded by laminin. (B) Muscle reconstruction by transplanted human cells was demonstrated by the detection of human-specific laminin-alpha 2. (C) The proportion of myofibers containing human nuclei at 4, 12, and 24 weeks after transplantation. (D) The proportion of myofibers containing human nuclei in reinjured (3+1 weeks) and in non-reinjured mice (4 weeks) at 4 weeks after transplantation. In C and D, data are presented as the mean ± standard deviation. (E) Distribution of the transplanted cells at 24 weeks after transplantation. Typical central nuclei of human origin were observed (outlined arrowheads). Some human cells located within the lamina rara beneath the basal lamina, indicating engraftment of the transplanted cells into a satellite cell compartment (white arrowhead). (F) Triple-staining for human Lamin A/C, PAX7, and pan-Laminin clearly demonstrated the existence of PAX7-positive human nuclei indicating the transplanted cells engrafted as satellite cells (white arrowhead). Human lamin A/C-negative host satellite cells were also detected (outlined arrowhead). Laminin was stained by a polyclonal antibody that recognizes both human and murine laminin, and was subsequently visualized with fluorescein isothiocyanate (FITC) (Green); human lamin A/C and human-specific laminin, with Cy3 (red). Nuclei were counterstained with DAPI (blue). Scale bars = (A) 100 µm, (B) and (E) 50 µm.