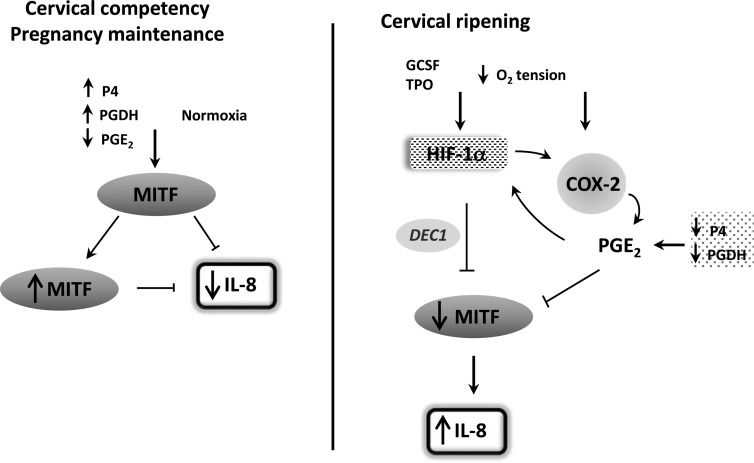
Fig. 12.
Model in which hypoxia and HIF-1α play important roles in cervical ripening. During most of gestation, the cervix is under normoxic conditions and serves to protect and maintain pregnancy. Increased progesterone (P4) up-regulates PGDH to decrease PGE2 levels in the cervix. MiTF-CX levels are increased and maintained in an autoregulatory feedback loop. MiTF-CX represses IL-8 gene expression in cervical stromal cells. During cervical ripening before labor, HIF-1α is stabilized in cervical stromal cells either through decreased O2 tension or increases in pseudohypoxia, factors such as GCSF and TPO. HIF-1α activates DEC1, leading to loss of MiTF-CX. Hypoxia and hypoxia mimetics that stabilize HIF-1α also result in induction of COX-2 and prostaglandin production, which further compromises MiTF-CX gene expression. Local progesterone metabolism and decreased PGDH contribute to up-regulated levels of PGE2. As in other cell types, PGE2 may also lead to further stabilization of HIF-1α. Ultimately, repression of IL-8 is lost leading to increased IL-8 production and infiltration of immune cells that further amplify IL-8 through IL-8 receptors on cervical stromal cells, neutrophils, monocytes, and macrophages.