Figure 2.
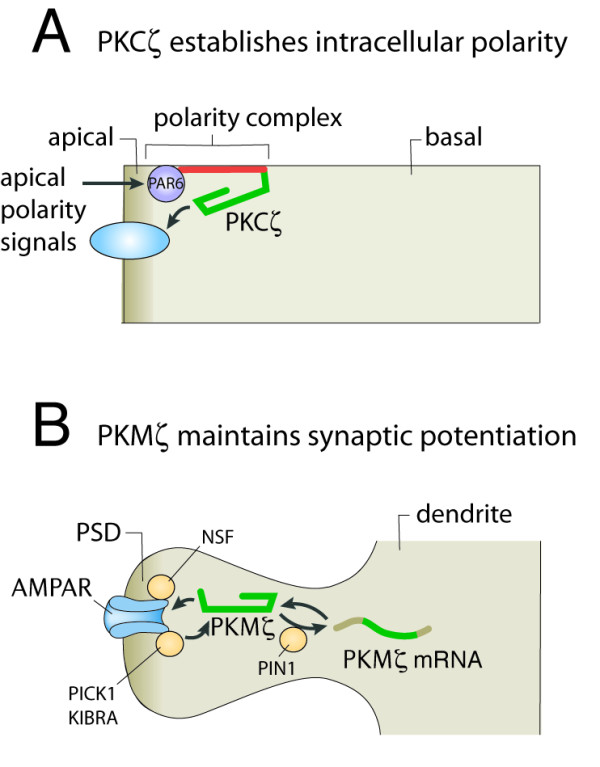
Model of PKMζ-mediated LTP maintenance as a specialized form of aPKC regulation of cell polarity.A) In polarized cells such as epithelial cells, polarity signals activate PAR6, which binds to the aPKC regulatory domain (red) and activates the enzyme. Phosphorylation by aPKC then traffics membrane proteins to the apical compartment of the polarized cell. B) In spines, PKMζ is synthesized after LTP induction or learning and potentiates synaptic strength by NSF-dependent trafficking of AMPARs to the PSD, the apical compartment of the postsynaptic spine. The absence of a PKCζ regulatory domain isolates PKMζ from other postsynaptic signaling, allowing the kinase to store long-term information without interference from short-term synaptic events. PKMζ maintains both synaptic potentiation and its own localization at the synapse by forming positive feedback loops, involving binding of PKMζ to postsynaptic GluA2 subunit-containing AMPAR-binding proteins, such as PICK1 and KIBRA. The persistent activity of postsynaptic PKMζ is required to maintain decreased AMPAR endocytosis, preventing both AMPAR and kinase elimination from the potentiated synapse. Other positive feedback loops, such as that involving PIN1, maintain increases in the amount of PKMζ through enhanced local translation.
