Table 2.
Oxyarylation of Homoallylic Alcohol [a]
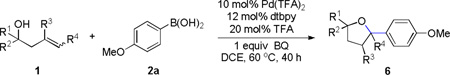 | |||
|---|---|---|---|
| Homoallylic alcohol | Tetrahydrofuran | Yield (%) [b] |
|
| 1 | 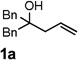 |
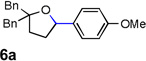 |
75 |
| 2 |  |
 |
65 |
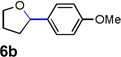
| 3 | 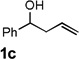 |
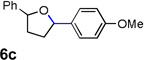 |
74 [c] |
| 4 | 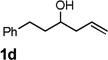 |
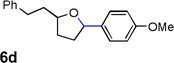 |
80 [d] |
| 5 | 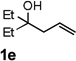 |
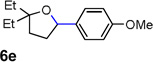 |
72 |
| 6 | 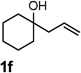 |
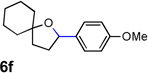 |
70 |
| 7 | 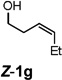 |
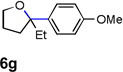 |
52 |
| 8 | 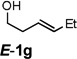 |
 |
61 |
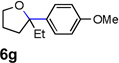
| 9 | 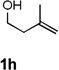 |
 |
31 [e] |
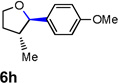
| 10 |  |
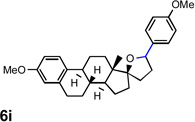 |
63 [f] |
| 11 | 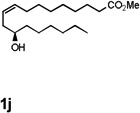 |
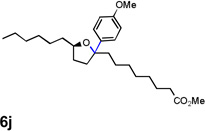 |
40 [g] |
Entries 2,7,8 and 9 were conducted with 0.2 mmol homoallylic alcohol 1; other entries were conducted with 0.1 mmol homoallylic alcohol 1.
Isolated yield.
d.r. 1.2:1.
d.r. 1.4:1.
trans: cis = 16:1.
d.r. 1.3:1.
d.r. 1.3:1.
