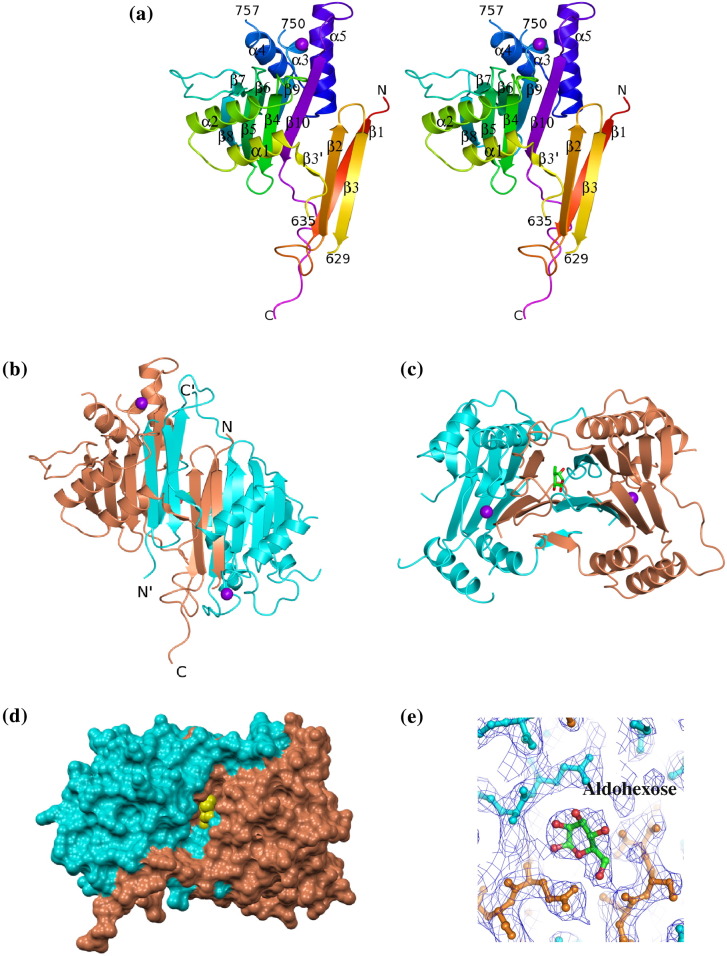
Fig. 3.
The structure of SpoIIE(590–827). (a) Stereo ribbon representation of the SpoIIE(590–827) phosphatase domain fragment. Chain B is color ramped from its N-terminus (red) to its C-terminus (magenta). The secondary structure elements are labeled, and the positions of breaks in the chain tracing are numbered. Mn2+ is represented as a purple ball. The detachment of the β1–β2–β3 segment from the rest of the structure is evident. (b–e) The domain-swapped dimer in approximately orthogonal views (b and c) and in space-filling representation (d). The two subunits are colored cyan and coral, and the chain termini are labeled in (b) and distinguished by the use of an apostrophe ('); Mn2+ are shown as purple balls. The more intimate association of the β1–β2–β3 segment with the partner protomer is evident. A cavity is formed at the subunit interface (d), and this is occupied by electron density (e) that cannot be accounted for by protein atoms; an aldohexose sugar has been modeled. As this sugar sits on a pseudo-2-fold axis, two half-occupancy molecules have been modeled, one of which is shown here. The origin of the sugar, if it is a sugar, is unknown. The sugar is shown in yellow space-filling format in (d), in cylinder format colored by atom in (b) and (c) and in ball-and-stick format in (e).