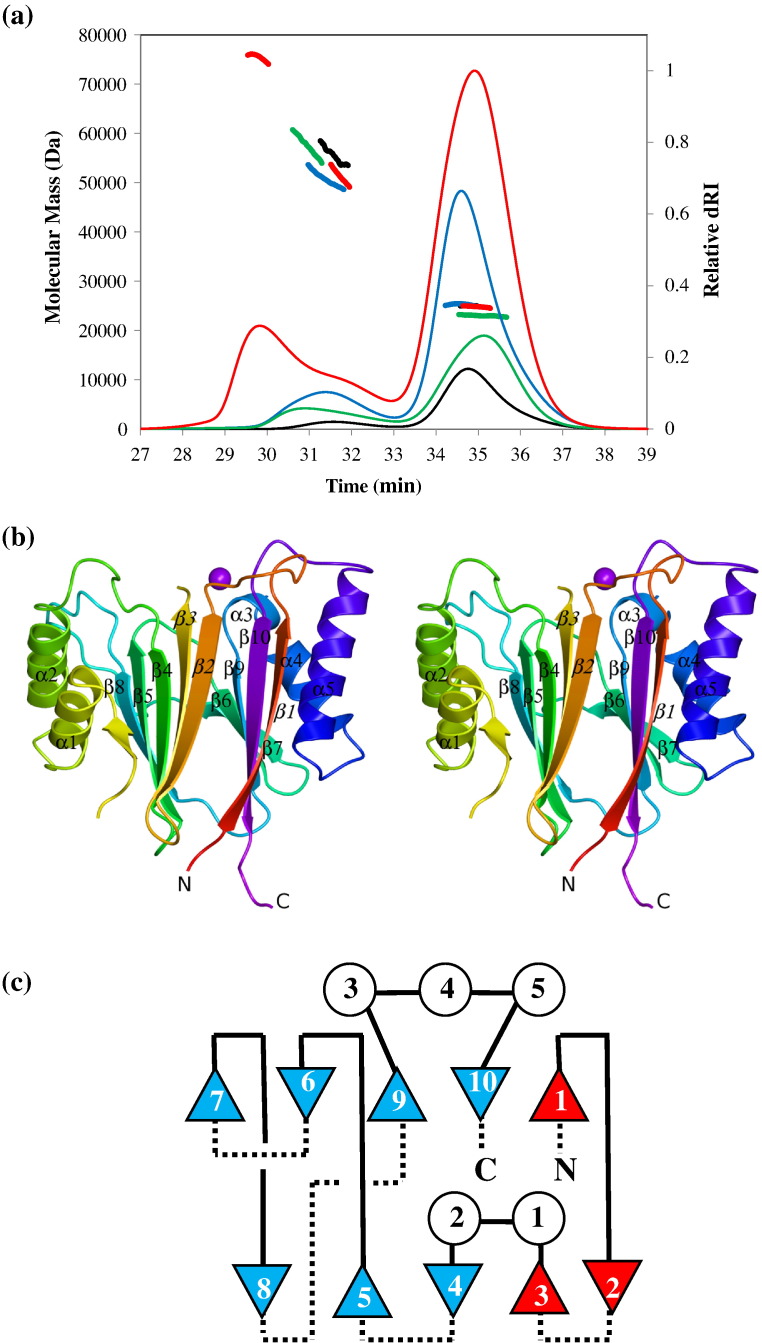
Fig. 4.
The PP2C domain of SpoIIE is a monomer. (a) SEC-MALLS traces of the molecular mass and differential refractive index (dRI) versus time. The thinner lines trace the absorbance at 280 nm of the eluate from a Superdex 10/300 S200 GL column as a function of time. The thicker lines represent the weight-average molecular weight of the species in the eluate calculated from refractive index and light-scattering measurements. SpoIIE(590–827) protein from two different preparations was loaded onto the column at 1 mg ml− 1 (green and black) and 5 mg ml− 1 (red and blue). In all four chromatograms, the principal species present has a molecular mass of 23–27 kDa, close to the expected mass of SpoIIE(590–827) of 26,507 Da. There is evidence of higher-molecular-weight material possibly representing PP2C domain dimers. (b) Stereo ribbon representation of the structure of the SpoIIE(590–827) PP2C monomer derived from the domain-swapped dimer. The ribbon is color ramped from its N-terminus (red) to its C-terminus (magenta). The secondary structure elements are labeled. Strands β1–β3 are labeled in italics to indicate that these strands are swapped in the dimer. (c) Topology diagram of the monomeric SpoIIE–PP2C domain in which strands are shown as triangles and helices are shown as circles. The continuous and broken lines distinguish connections across the top and across the bottom of the β-sheets, respectively. The red symbols denote β-strands that are swapped in the dimer.