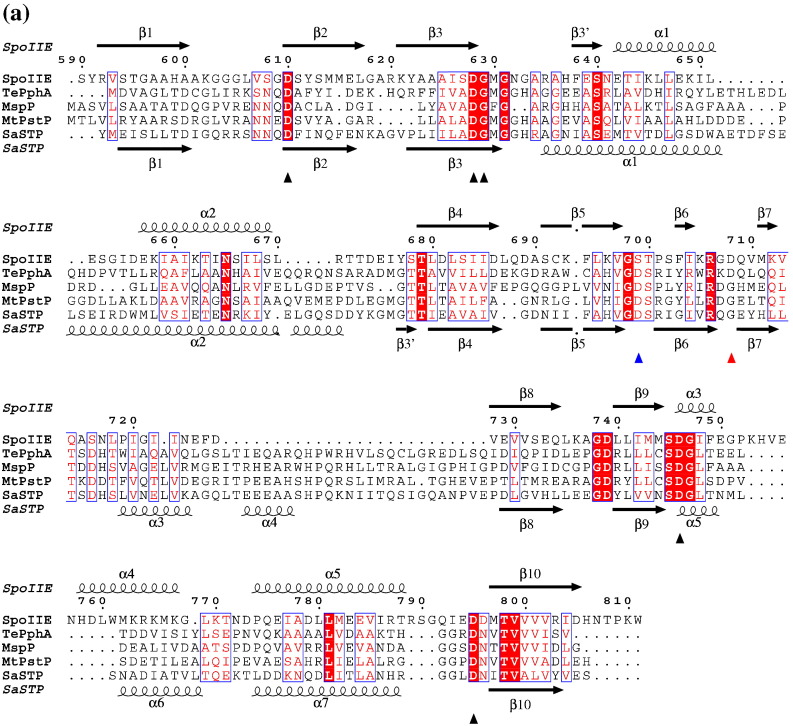
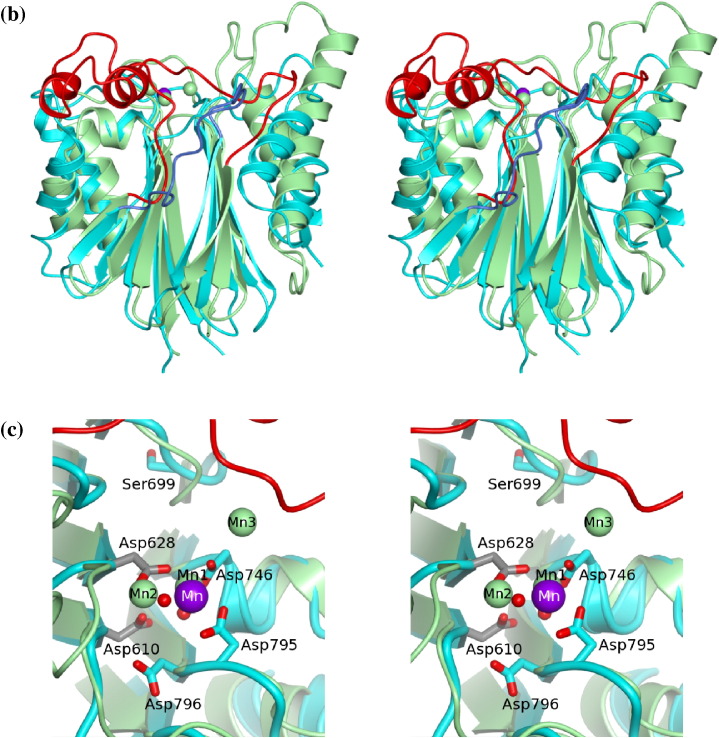
Fig. 5.
Structural and sequence comparisons among PP2C phosphatases. (a) Sequence alignment of the PP2C domain of SpoIIE with those of PphA from T. elongatus, MspP from M. smegmatis, PstP from M. tuberculosis and STP from S. agalactiae. The secondary structure elements from the structures of SpoIIE(590–827) and SaSTP are shown above and below the alignment, respectively. The residue numbering refers to SpoIIE(590–827). The triangular symbols refer to residues discussed in the main text. (b) Stereo overlay of the structures of SpoIIE(590–827) and MtPspP represented as cyan and light-green ribbons, respectively, with Mn shown as spheres. There is a very close superposition of the β-sheet core with the principal structural deviations occurring in the loop and helical regions. The flap segment shown in blue for SpoIIE is much shorter than that in MtPspP shown in red. (c) The single bound metal in SpoIIE (purple) and the three bound metals in MtPspP (light green) with protein chains colored as in (b). The side chains of a cluster of Asp residues and two metal-coordinating water molecules, one of which is eclipsed by the metal, are displayed for SpoIIE. The side chain of Ser699, which takes the place of one of the Mn3-coordinating Asps in the other PP2C domains, is also shown oriented away from the metal coordination site. The differently colored carbons of Asp610 and Asp628 emphasize their origin in the domain-swapped subunit.