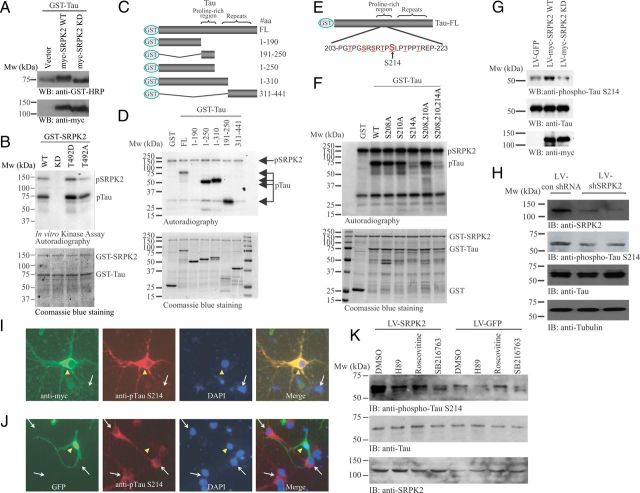
Figure 4.
Tau is the physiological substrate of SRPK2. A, Wild-type SRPK2 affects the phosphorylation of tau in HEK293 cells. GST-Tau was co-transfected with empty vector, myc-SRPK2 WT, or myc-SRPK2-KD into HEK293 cells. Forty-eight hours after transfection, cell lysates were collected and analyzed by Western blot with anti-GST-HRP or anti-myc antibody. Migration of tau was slower in myc-SRPK2 WT overexpressed sample (top). Expression of the SRPK mutants were also verified (bottom). B, SRPK2 phosphorylates tau in vitro. Bacterial expressed and purified GST-Tau was incubated with GST-SRPK2 WT, GST-SRPK2 KD, GST-SRPK2 T492D, or GST-SRPK2 T492A in the presence of [γ32P]-ATP. The reaction products were separated in SDS-PAGE and analyzed by autoradiography (top). The protein inputs were shown using Coomassie Blue staining (bottom). C, D, SRPK2 phosphorylates tau on the proline-rich region. Various tau truncates (C) were bacterially expressed, purified, and incubated with recombinant GST-SRPK2 in the presence of [γ32P]-ATP. The reaction products were separated in SDS-PAGE and revealed by autoradiography (D). SRPK2 dramatically phosphorylates the tau truncates containing the proline-rich region. The protein inputs were also shown. E, Schematic diagrams of the potential SRPK2 phosphorylation sites in tau. F, SRPK2 phosphorylates tau on S214 residue. Various GST-tagged tau point mutants were bacterially expressed, purified, and then incubated with recombinant GST-SRPK2 in the presence of [γ32P]-ATP. The reaction products were separated in SDS-PAGE and the phosphorylation was visualized by autoradiography (top). The proteins input were analyzed by Coomassie Blue staining (bottom). G, Overexpression of SRPK2 upregulates tau phosphorylation on S214. Primary neurons were infected with LV-GFP, LV-myc-SRPK2 WT, or LV-myc-SRPK2 KD for 72 h. Tau phosphorylation was monitored using anti-phospho-Tau S214 antibody (top). Expressions of Tau (middle), SRPK2 WT, and SRPK2 KD were verified by anti-myc antibody (bottom). H, Depletion of SRPK2 reduces tau phosphorylation on S214. Primary neurons were infected with lentivirus-expressing control shRNA (LV-con shRNA) or shSRPK2 for 48 h. The cell lysates were resolved by SDS-PAGE, and the knockdown efficiency was examined using anti-SRPK2 antibody (first panel). Reduced expression of SRPK2 also inhibited tau phosphorylation on S214 (second panel). The expressions of total tau (third panel) and tubulin were also shown (fourth panel). I, Overexpression of SRPK2 in primary neuron induces tau phosphorylation on S214. Primary mouse neurons (P1) were infected with LV-myc-SRPK2 at DIV7 for 72 h. After fixation in 4% paraformaldehyde, the cells were costained with anti-myc and anti-phospho-Tau S214 antibodies. By comparing with the non-infected cells (white arrow), overexpression of SRPK2 (yellow triangle) significantly enhanced the phosphorylation of tau on S214. J, Depletion of SRPK2 inhibited tau phosphorylation on S214. Primary mouse neurons (P1) were infected with adenovirus expressing shSRPK2 at DIV7 for 72 h. The cells were fixed and immunostained with anti-phospho-Tau S214 antibody. Phosphorylation of tau S214 was reduced in the virus-infected cells (yellow triangle) when compared with the non-infected cells (white arrow). K, SRPK2-induced tau S214 phosphorylation is implicated with GSK3. Cortical neurons (DIV7) were infected with LV-GFP or LV-SRPK2 WT for 48 h, followed by 10 μm H89 (PKA inhibitor), roscovitine (CDK5 inhibitor), or SB216763 (GSK3 inhibitor) stimulation for 6 h. Phosphorylation of tau S214 was then monitored by immunoblotting.