Figure 6.
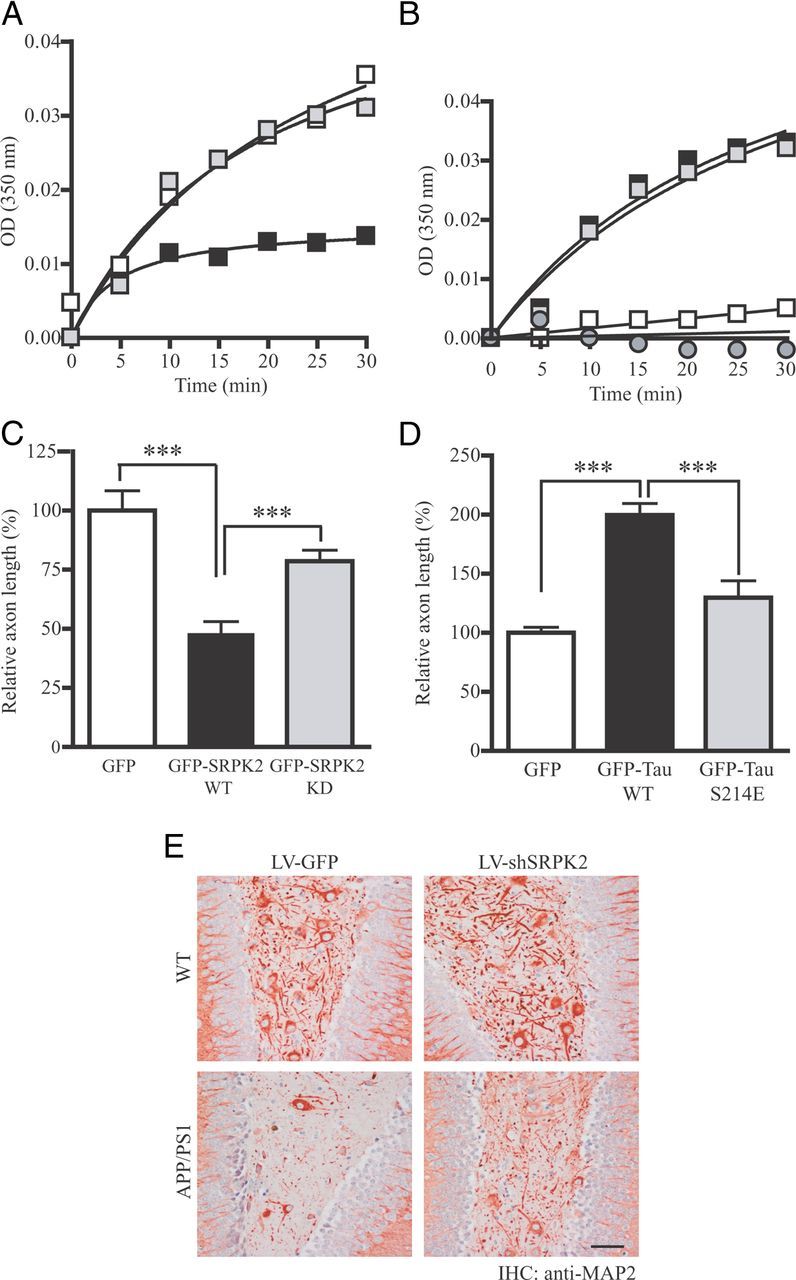
SRPK2 inhibits tau-dependent microtubule polymerization and neurites outgrowth. A, SRPK2 phosphorylation inhibits tau-promoted microtubule polymerization. Recombinant GST-Tau or GST-Tau S214A was first incubated with purified GST or GST-SRPK2 to promote tau phosphorylation. The reaction products were then incubated with purified bovine brain tubulin. The formation of microtubule was monitored by measuring the absorbance in a spectrophotometer at 350 nm (open square: Tau WT + GST; solid square: Tau WT + SRPK2; gray square: Tau S214A + SRPK2). B, Tau S214E mutant fails to promote microtubule polymerization in vitro. GST, GST-Tau, GST-Tau S214A, or GST-Tau S214E recombinant protein was incubated with purified bovine brain tubulin. The turbidity of polymerized microtubule was measured at 350 nm (open square: GST; solid square: GST-tau; gray square: GST-Tau S214A; gray circle: GST-tau S214E). C, SRPK2 represses axon elongation in primary neurons. Primary neurons (DIV 7) were transfected with GFP, GFP-SRPK2 WT, or GFP-SRPK2 KD for 24 h. The cells were then fixed and immunostained using anti-Tau-1 antibody to display the axons. The lengths of the primary neurites were measured. Expression of SRPK2 significantly inhibited the axonal growth of primary neuron (***p < 0.001, Student's t test, n = 20). Scale bar, 200 μm. D, The ability of tau to promote axon elongation in primary neuron was decreased by S214E mutation. Primary neurons (DIV 7) were transfected with GFP, GFP-Tau, or GFP-Tau S214E for 24 h. The cells were then fixed and the images of the transfected neurons were captured under fluorescent microscope. Overexpression of wild-type tau significantly enhanced the axonal elongation (***p < 0.001, Student's t test, n = 20). Scale bar, 200 μm. E, Increased neurite density in SRPK2-depleted APP/PS1 mice. The dendrites of cells in various virus-infected DG were stained using anti-MAP2 antibody. Scale bars: 50 μm.
