Abstract
Accelerated telomere length attrition has been associated with psychological stress and early adversity in adults; however, no studies have examined whether telomere length in childhood is associated with early experiences. The Bucharest Early Intervention Project is a unique randomized controlled trial of foster care placement compared with continued care in institutions. As a result of the study design, participants were exposed to a quantified range of time in institutional care, and represented an ideal population in which to examine the association between a specific early adversity, institutional care and telomere length. We examined the association between average relative telomere length, telomere repeat copy number to single gene copy number (T/S) ratio and exposure to institutional care quantified as the percent of time at baseline (mean age 22 months) and at 54 months of age that each child lived in the institution. A significant negative correlation between T/S ratio and percentage of time was observed. Children with greater exposure to institutional care had significantly shorter relative telomere length in middle childhood. Gender modified this main effect. The percentage of time in institutional care at baseline significantly predicted telomere length in females, whereas the percentage of institutional care at 54 months was strongly predictive of telomere length in males. This is the first study to demonstrate an association between telomere length and institutionalization, the first study to find an association between adversity and telomere length in children, and contributes to the growing literature linking telomere length and early adversity.
Keywords: telomere, institutionalization, early childhood, gender
Introduction
Exposure to adversity and stress has consistently been associated with a range of negative health outcomes including psychological disorders, cardiovascular disease and immunologic disorders.1,2 Emerging evidence indicates that early childhood may represent a particularly vulnerable time period, as the brain is undergoing rapid neurodevelopmental changes.3,4 Although the link between a range of negative outcomes and adversity is established, the mechanism of how these early experiences alter biological processes has yet to be fully elucidated. Biomarkers of adversity, such as alterations in the hypothalamic-pituitary adrenal axis and the autonomic nervous system, have been identified and offer insight into this process at a systems level.5-9 Telomere shortening, a marker of biological aging, may represent an additional cellular level biomarker of adversity.
Telomeres are specialized nucleoprotein complexes located at the end of chromosomes that promote chromosomal stability. Telomeres are required due to the ‘end DNA replication problem’ where DNA polymerase can only replicate DNA in the 5′ to 3′ direction. In germ cells and stem cells, a cellular enzyme, telomerase, functions to extend telomeres. However, telomerase is not present in the majority of somatic cells and therefore telomere length shortens with each successive cellular division. Cellular damage due to increased oxidative stress or DNA damage due to environmental exposures can further accelerate telomere shortening. Once telomere length reaches a critical point, cellular senescence is triggered, cell division ceases and the cell eventually dies.10 Accelerated telomere length shortening has been associated with normative aging and linked to similar negative health outcomes as exposure to early adversity, including cardiovascular disease, diabetes and cognitive decline.11,12 Certain environmental factors have been associated with shortening of telomere length, including cigarette smoking,13 radiation,14 oxidative stress15,16 and most recently, psychological stress including a history of early maltreatment, mood disorders, self-reported psychological stress and stress exposure related to being the caretaker of a chronically ill individual.17-23 These studies suggest that the acceleration of the cellular aging process occurs with psychological distress and this may represent one mechanism by which early adversity is translated into increased morbidity and mortality across health indices.
Two previous studies have demonstrated an association between retrospective reports of childhood adversity and adult telomere length.18,19 In one study of 31 healthy adults without psychiatric disorders, those with a self-reported history of emotional or physical neglect had significantly shorter telomere length than individuals with no history of maltreatment.18 In a larger case control study of adults with and without anxiety disorders, across both case and control subjects, childhood adversity was significantly associated with shorter relative telomere length.19 However, no previous studies have examined whether this association can be demonstrated in children. Early childhood likely represents a critical period for the interaction between stress, cellular aging and neurodevelopment for several reasons. First, brain development is rapid over the first years of life. Further, early childhood is both a period of rapid telomere attrition and the putative time point at which an individual’s rate of telomere length attrition is established epigenetically.24,25 Clarification of a temporal relation between adversity exposure and cellular level biological changes would represent a significant advancement for the study of early life stress.
Children living in institutions represent a well-studied model of early adversity. These children receive little attention to their individual needs, are exposed to low-quality caregiving and have limited opportunities to form selective interpersonal attachments. The detrimental impact of institutionalization across biological, social, emotional, neurological and cognitive domains has been established in multiple studies over the last 50 years.26,27 Although improved caregiving environments associated with either adoption or placement in foster care may mitigate some detrimental outcomes, recovery is not uniform, and in some domains, children remain at risk.28,29 Multiple factors likely have a role in recovery, including the total amount of exposure to institutionalized care, the age at which the child is placed in the institution, prenatal experiences, selection bias for adoption and genetic factors.27,30,31
The Bucharest Early Intervention Project is the only longitudinal randomized controlled trial of foster care compared with continued institutional care ever conducted. Children in this study were recruited from one of six institutions in Romania and randomly assigned to either the care as usual group (CAUG) or the foster care group (FCG) arm of the clinical intervention. At the initial assessment, children had spent a significant, but varying, proportion of their early lives in institutional care, and we report on our findings related to percent institutional care at baseline and 54 months of age.
In the current study, we hypothesized that the cumulative amount of time a child, early in their life, spent in institutional care would be inversely related to telomere length in middle childhood. We hypothesized that children who had spent a greater percentage of their early life in institutional care would have significantly shorter telomere length than children who had less exposure to institutional care. Given that gender differences exist in adult telomere length and in stress response, we also examined whether gender would moderate the impact of time in institutional care.32-36
Methods
Participants
Participants were children enrolled in the Bucharest Early Intervention Project,37 a longitudinal randomized controlled trial of foster care compared with institutional care in Romanian described in detail elsewhere.26,38 A total of 136 children were enrolled, between 6 and 30 months of age, who were currently residing in one of six different institutions in Bucharest, Romania. Following baseline assessments, 68 of the children (33 males and 35 females) were randomly assigned to CAUG and 68 (34 males and 34 females) were randomly assigned to FCG. The establishment of the foster care system created for this project as an intentional alternative to institutional care and the ethical considerations related to this project have been described in detail.39,40 This study was approved by the Institutional Review Boards of Children’s Hospital of Boston, University of Maryland and Tulane University.
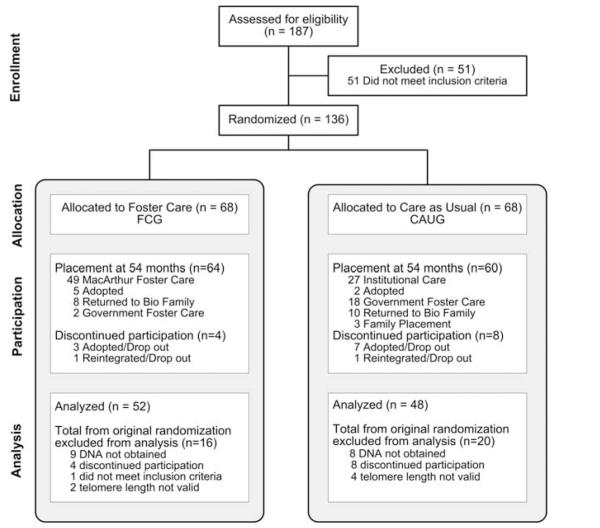
Following randomization and placement of children in foster care, all subsequent decisions regarding placement were made by the child protection commissions in Bucharest in accordance with Romanian law, with the exception that no child removed from an institution and placed in the newly created foster care could be returned to the institution.39 Children were exposed to different amounts of time in institutional care as a result of three factors. First, not all children entered the institution at birth as some children resided with their biological families for varying amounts of time before placement in the institutions. Second, although the randomization process occurred at a single time point (baseline), the placement of children into foster care homes was not immediate, as families needed to be identified and trained before the child entering the foster home. Third, during the course of this longitudinal study, some children in both groups were moved into other types of care including adoption, government createdand sponsored foster care or returned to their biological families. Careful life histories on each child were maintained until 54 months of age (Figure 1). Although previous analyses of these children examined outcomes based on initial randomization (intent to treat), our hypothesis that telomere length would reflect the total amount of exposure to institutional care required that we examine the relation between the individual child’s percent of time in institutional care, as this most accurately reflects their exposure to this adverse environment. Baseline and 54-month time points were specifically chosen to provide a range of exposure and in consideration of findings demonstrating rapid telomere length change from birth to age 4.41,42
Figure 1.
Flow of participants in the Bucharest Early Intervention Project: ever institutionalized. This consort diagram presents the placement and movement of the participants in the Bucharest Early Intervention Project study, from assessment through 54 months of age. Although original group designation at baseline to either continued institutional care (CAUG) compared with newly created MacArthur foster care (FCG) was random, note that significant placement changes occurred in both groups by 54 months of age.
Measures
Relative telomere length
DNA, obtained between 6 and 10 years of age, was extracted using standard protocols, from buccal swabs collected in Romania and shipped to the United States. The average relative telomere length, as represented by the T/S ratio, was determined by quantitative real-time PCR using a 7900HT Thermocycler (Carlsbad, CA, USA) (Applied Biosystems) in a 384-well format. Briefly, 5 ng of genomic DNA extracted from buccal cells was dried down in a 384-well plate and resuspended in 10 μl of either the telomere or 36B4 PCR reaction mixture (Qiagen, Valencia, CA, USA). The telomere reaction mixture consisted of 1×Qiagen Quantitect Sybr Green Master Mix (Qiagen, Valencia, CA, USA), 2.5 mm of dithiothreitol, 270 nm of Tel-1 primer (GGTTTTTGAGGGTGAGGGTGAGGGTGAGGGTGAG GGT) and 900 nm of Tel-2 primer (TCCCGACTA TCCCTATCCCTATCCCTATCCCTATCCCTA). The reaction proceeded for one cycle at 95 °C for 5 min, followed by 40 cycles at 95 °C for 15 s and 54 °C for 2 min. The 36B4 reaction consisted of 1×Qiagen Quantitect Sybr Green Master Mix, 300 nm of 36B4U primer (CAGCAAGTGGGAAGGTGTAATCC) and 500 nm of 36B4D primer (CCCATTCTATCATCAAC GGGTACAA). The 36B4 reaction proceeded for one cycle at 95 °C for 5 min, followed by 40 cycles at 95 °C for 15 s and 58 °C for 1 min 10 s. All samples for both the telomere and single-copy gene (36B4) reactions were performed in triplicate on different plates in the same well position. Interplate coefficients of variations for the threshold cycle (Ct) values were below 1% for both the telomere and single gene reaction. In addition to the samples, each 384-well plate contained a 6-point standard curve from 0.625 ng to 20 ng, using pooled buffycoat-derived genomic DNA. The slope of the standard curve for both the telomere and 36B4 reactions was −3.4±0.1, and the coefficient of determination (R2) value for both reactions was 0.98 and 0.99, respectively.43 The exponentiated T/S ratio (−dCt) for each sample was calculated by subtracting the average 36B4 Ct value from the average telomere Ct value, then 2(−dCt). Telomere length was examined as both the ratio of repeats to single copy (T/S ratio) and as a normalized z-transformed variable. Samples with inconsistent replicates and outliers were removed from analysis. Median length was 10.87±6.24.
Percent time institution
Life histories documented month-by-month placements for each child from study entry through 54 months of age, when the formal intervention ended. Thus, the percent of time spent in institutional care was calculated on the basis of the number of days in institutional care compared with the number of days the child spent in other environment placements (including biological family, foster care, adopted family) before baseline assessment and again through 54 months of age. Because there was significant movement of children between baseline assessment and 54-month assessment in both the FCG and the CAUG, the percentage of time each individual child spent in institutional care varied at each time point. As the percent of time the child spent in institutional care, at baseline and 54 months, is the most accurate characterization of total exposure to early institutional care, this was used to predict relative telomere length in middle childhood.
Covariates
Additional potential confounders or predictors of telomere length were considered and controlled for in regression models. These included: group assignment (FCG, CAUG), gender, ethnicity (Romanian versus other), low birth weight (yes/no, < 2500 kg) and age at the time of buccal swab collection.
Data analysis
Data were examined by calculating the percent of time a child had been institutionalized before baseline assessment and again at the 54-month assessment time point. These percentages were examined in relation to telomere length (T/S ratio) in middle childhood. Bivariate relationships were examined through basic correlations (Pearson or Spearman, where appropriate) and one-way analysis of variance. Bivariate tests were used to confirm that there was an association between percent time institutionalized and telomere length, and between telomere length and institutionalization and other covariates. Given the suspicion of heteroskedasticity and the presence of outliers, adjusted robust linear regressions were run as an alternative to ordinary least squares regression to examine the relations between percent time spent in institution and telomere length after accounting for potential confounders, including intervention group, gender, birth weight and age at telomere data collection. Two-way interactions were also examined for percent time institutionalized and gender, age and group.
Sample characteristics are presented in Table 1. Valid telomere length was obtained on 100 of 109 participants, 48 from the CAUG group and 52 from the FCG group. In the final analysis, there were 41 females and 59 males. Ethnicity and further demographic variables are presented in Table 1.
Table 1.
Characteristics of participants according to telomere length in middle childhood (N = 109)
| N (% or median ± s.d.) |
Median telomere length (T/S ratio ± s.d.) |
|
|---|---|---|
| Sex | ||
| Male | 62 (56.9%) | 8.8 ±6.1 |
| Female | 47 (43.1%) | 9.9 ±6.4 |
| Ethnic | ||
| Romanian | 60 (55.1%) | 8.8 ±5.9 |
| Romanian/other/ unknown |
49 (44.9%) | 10.2 ± 6.6 |
| Low birth weight | ||
| <2500 kg | 36 (33.0%) | 10.0 ±6.7 |
| ≥2500 kg | 73 (67.0%) | 8.8 ±5.9 |
| Age at telomere data collection | ||
| 6 to <8 years | 34 (31.2%) | 9.5 ± 6.4 |
| 8 to > 9 years | 47 (43.1%) | 8.8 ±6.0 |
| 9 to 10 years | 28 (25.7%) | 10.0 ±6.5 |
NS, not significant.
aAll values NS >0.05.
Results
Association between early social deprivation and telomere length in middle childhood
A significant inverse association was detected between the percent of time in institutional care and telomere length in middle childhood. A greater percent time in the institution at baseline and at 54 months of age was associated with shorter telomere length in middle childhood (χ2-value from crude robust regression for percent time at baseline = 6.20, P = 0.0128 and at 54 months = 3.98, P = 0.0420).
The results of final multivariate models examining the association between telomere length in middle childhood and the percent time spent in institutional care at baseline (Model 1) and 54-months (Model 2) are presented in Table 2. Percent time spent in the institution at baseline (β = −0.07027, standard error = 0.03092) and 54 months (β = −0.0500, standard error = 0.0294) remained significantly and inversely associated with telomere length, even after accounting for group assignment (FCG, CAUG), gender, ethnicity, low birth weight and age at telomere collection. The greatest proportion of variance in telomere length was explained by the percent time spent in institutionalized care at baseline (R2 = 12.2%).
Table 2.
Early and cumulative environmental stress exposure and telomere length in middle childhood – results from adjusted multivariate models, with and without effect modificationa,b
|
β (standard error) |
||
|---|---|---|
| Model 1 | Model 2 | |
| Percent time institution at baseline | −0.07027‡ (0.03092) | |
| Percent time institution at 54 months | −0.0500† (0.0294) | |
| R2 | 12.20% | 8.89% |
|
| ||
| Gender modification: | Girls (n = 41) | Boys (n = 59) |
|
| ||
| Percent time institution at baseline R2 | −0.1294 ‡ (0.0395) 26.9% | −0.0207 (0.0410) 7.0% |
| Percent time institution at 54 months | −0.0061 (0.036) | −0.0950‡ (0.0364) |
| R2 | 6.2% | 14.3% |
Abbreviations: ANOVA, analysis of variance, CAUG, care as usual group; FCG, foster care group; R2, coefficient of determination.
Adjusted for group (CAUG versus FCG), gender (in non-stratified models), ethnicity, age at telomere data collection and low birth weight.
P-value:
<0.05 or
<0.10, based on ANOVA with robust regression.
Modification by gender
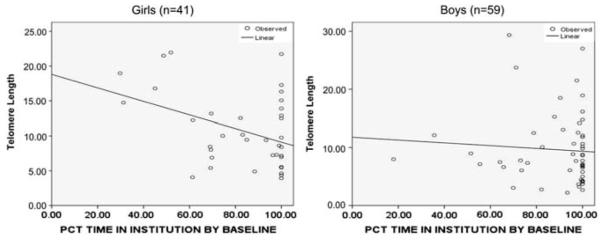
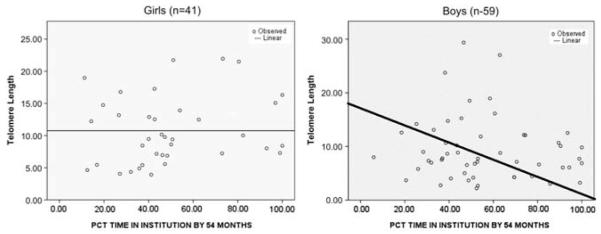
Contrary to our initial hypothesis, no significant difference in telomere length by gender was detected. Although the percentage of institutional care at neither time point was statistically different between genders, we detected a significant gender modification on the relationship between telomere length and institutional exposure. The gender differences in the percent of time spent in institution at baseline is depicted in Figures 2 and 3.
Figure 2.
Percent institution at baseline by gender. Adjusted robust regression line plotted to demonstrate the association between percent of time a child had been in institutional care at baseline, by gender and telomere length.
Figure 3.
Percent institution at 54 months by gender. Adjusted robust regression line plotted to demonstrate the association between percent of time a child had been in institutional care at 54 months of age, by gender and telomere length.
Among females, the percent of time spent in the institution at the baseline assessment was significantly associated with later T/S ratio, whereas total percent of time in institutions at the 54-month assessment was not significant. For boys, the opposite was true. That is, percent of institutionalization at baseline was not predictive of subsequent T/S ratio, whereas the total percent time of institutionalization through 54 months was associated with T/S ratio in middle childhood (Table 2). Baseline percent institution explained a substantial proportion of the variance in telomere length in middle childhood for females, with an adjusted R2 of 26.9%. Among females, 1% percent time increase in institutionalized care at baseline was associated with an average 0.13 unit declines in T/S ratio. Therefore, a female child, who at baseline had spent 50% of her life in the institution, would have an approximately 12-unit decrease in relative telomere length in middle childhood compared with a child who had spent 30% of her time in the institution. Among males, percent institutional care at 54 months of age, rather than percent at baseline, explained a significant proportion of the variance in T/S ratio in middle childhood. Although this impact was not as large as seen with females at baseline, it was significant with an adjusted R2 of 14.3%. Therefore, it appears that the timing of exposure to institutional care and its relation to telomere length differs between the genders. For females, exposure before baseline assessment was most predictive of later telomere length, whereas for males, the total amount of institutionalization at 54 months was most predictive of telomere length in middle childhood.
Discussion
In this study, we demonstrated in Romanian children an association between shorter relative telomere length in middle childhood and increased exposure to early institutional care. These results offer further support for the hypothesis that alterations in cellular aging may be one pathway by which early adversity impacts health outcomes.17,20,22
These findings complement recent data that early childhood experiences, including abuse, childhood adversity and childhood serious illness, are associated with shorter relative telomere length in adults. This study extends these findings to include adversity as a result of exposure to the deprivation associated with early institutional rearing and is the first study to demonstrate that the association between adversity and telomere length is detectable during childhood.19,44 Our findings are also consistent with studies that demonstrate that epigenetic alterations, such as methylation, are associated with other forms of early adversity including child abuse and prenatal maternal depression.45-50 Interestingly, in longitudinal studies examining methylation, early childhood appears to be a time period of more extensive epigenetic remodeling.42,51,52 Because epigenetic regulation of telomere length also is established in early childhood, these findings raise an intriguing and testable hypothesis. That is, early adversity results in widespread changes in methylation 53 that affect both gene specific regulation and telomere length regulation, thereby resulting in an increased risk due to not only altered gene regulation, but also decreased telomere length. Although later environmental changes, such as placement in an enriched environment, can occur, these may or may not alter established epigenetic patterns. Reduced telomere length, and perhaps a greater rate of telomere length decline over time due to epigenetically established regulation of telomere length attrition, may persist even after the adverse environment is ameliorated. If longitudinal studies of telomere length in children exposed to early adversity reveal that children exhibit more rapid reductions in telomere length, despite environmental enhancement, this may provide greater understanding for not only the lasting negative impact of early adversity across the lifespan, but also the delayed onset of some of the negative health consequences as a crtical telomere length difference may not be observable until later in life.8 Approaches to increase the plasticity of epigenetic regulation, as well as intensive focus on early interventions and exposure to enhanced early environments, would then become even more critical areas of research.
The second important contribution of this study is the identification of gender differences in the interaction between telomere length and institutional exposure at different time points. This finding adds to the other gender difference detected in the Bucharest Early Intervention Project study, where foster care was more effective in reducing psychiatric symptomatology and disorders in females than in males at 54 months of age.38 One hypothesis to explain these findings is the existence of a gender-specific sensitive period to early adversity. Alternatively, gender differences in stress responsiveness, and thus the impact of adversity at the cellular level, may exist. Although these gender findings clearly need to be replicated, support for gender differences in stress responsiveness have been demonstrated.54,55
Several limitations to this study exist. First, DNA was collected over a 3-year age range. Although all analyses controlled for the age at which telomere DNA was collected, future studies in children should examine telomere length within a reduced age range, though limited differences in telomere length during later childhood and adolescence has been demonstrated. Second, we had a limited ability to assess for prenatal exposures, which might be differentially associated with placement in the institution, as well as independently contribute to the differences in telomere length. To address this potential confounder, we controlled for birth weight as a proxy for prenatal health. Third, although we postulate that telomere length is related to the overall adversity associated with institutional care, we cannot exclude the possibility that the causative exposure was a particular aspect of their care that is highly correlated with percent time, such as nutritional status. Malnutrition has been associated with institutional care and postulated to be associated with the known deficits in physical growth demonstrated in institutionalized children.56 Thus, telomere shortening may not be due to, per se, the total cumulative exposure to severe psychosocial deprivation, but rather, exposure to a particular aspect of this type of experience. Future studies examining the association of telomere length in children with well-defined measurements of community, household and individual level exposures including nutrition, toxins and adverse life events may refine this relation. Still, our findings are in agreement with previous studies demonstrating associations between telomere length and childhood adversity providing support for our main hypothesis that the cumulative exposure of institutionalization is driving this association. Fourth, this is the first study to examine the association between early adversity and telomere length, using buccal swab DNA and not DNA extracted from peripheral blood. Although differences in telomere length between cell types (that is, between lymphocytes and epithelial cells) exist, there is also evidence to support that correlation exists between telomere length in different cell types, and a previous study has demonstrated that telomere length from buccal cell DNA is associated with increased risk of Alzheimer’s disease.57 The establishment of telomere length extracted from DNA samples obtained non-invasively as a valid biomarker of early experience, and a predictor of negative health outcomes, would represent a significant advancement for the field; thus, it is important to note that our findings are consistent with previous studies of the impact of early childhood events on telomere length in DNA from lymphocytes or peripheral blood mono-nuclear cell.
The biological significance of shorter telomere length in children is unknown. Although in adults, shortened telomere length is associated with increased rates of cardiovascular disease, elevated rates of cancer and cognitive decline, these associations have not been demonstrated in children. Few longitudinal studies of telomere length have been done and little is known about the ‘typical’ rate of telomere change for children, though studies have demonstrated a rapid decline in telomere length in early childhood.41,42 Future longitudinal studies of telomere length in children exposed to a range of adversities would be highly relevant.
Although evidence of the negative health impact of early and chronic toxic stress continues to accumulate, the biological mechanism by which psychological stress is translated into negative health outcomes is not yet well defined. Future studies are needed to examine the association between telomere length, institutional care and other outcomes including cognitive, psychological and neurobiological measures. It will be interesting to examine the rate of telomere length attrition longitudinally and determine whether early adverse experiences lead to a persistent accelerated rate of telomere decline or instead result in an initial ‘scar’ on telomere length without impacting the rate of telomere attrition over the life course. Clear evidence that early adversity has lasting negative health consequences exists; however, objective quantification of cumulative adversity exposure has been challenging. Telomere length may represent an objective epigenetic biomarker of early adversity and putatively one mechanism by which early adversity gets ‘under the skin’ and into our biology.
Acknowledgments
This research was supported by the Harvard Center for the Developing Child (Drury, Nelson), The John D and Catherine T MacArthur Foundation Research Network on ‘Early Experience and Brain Development’ (Charles A Nelson, PhD, Chair) and the Binder Family Foundation (Nelson), the Center for Training Research and Education at Tulane University School of Medicine (Drury), a NARSAD Young Investigator Award (Drury).
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- 1.McEwen B. Protective and Damaging Effects of Stress Mediators. N Eng J Med. 1998;338:171–179. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- 2.Phillips N, Hammen C, Brennan P, Najman J, Bor W. Early adversity and the prospective prediction of depressive and anxiety disorders in adolescents. J Abnorm Child Psychol. 2005;33:13–24. doi: 10.1007/s10802-005-0930-3. [DOI] [PubMed] [Google Scholar]
- 3.Green J, McLaughlin K, Berglund P, Gruber M, Sampson N, Zaslavsky A, et al. Childhood adversities and adult psychiatric disorders in the national comorbidity survey replication 1. Arch Gen Psychiatry. 2010;67:113–123. doi: 10.1001/archgenpsychiatry.2009.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stevens H, Leckman J, Copland J, Suomi S. Risk and resilience: early manipulation of macaque social experience and persistent behavioral and neurophysiological outcomes. J Am Acad Child Adolesc Psychiatry. 2009;48:114–127. doi: 10.1097/CHI.0b013e318193064c. [DOI] [PubMed] [Google Scholar]
- 5.Lupien S, King S, Meaney M, McEwen B. Can poverty get under your skin? Basal cortisol levels and cognitive function in children from low and high socioeconomic status. Dev Psychopathol. 2001;13:653–676. doi: 10.1017/s0954579401003133. [DOI] [PubMed] [Google Scholar]
- 6.McEwen B, Seeman T. Protective and damaging effects of mediators of stress: elaborating and testing the concepts of allostasis and allostatic load. AnnN Y Acad Sci. 1999;896:30–47. doi: 10.1111/j.1749-6632.1999.tb08103.x. [DOI] [PubMed] [Google Scholar]
- 7.Evans G. A multimethodological analysis of cumulative risk and allostatic load among rural children. Dev Psychol. 2003;39:924–933. doi: 10.1037/0012-1649.39.5.924. [DOI] [PubMed] [Google Scholar]
- 8.Shonkoff S, Boyce W, McEwen B. Neuroscience, molecular biology, and the childhood roots of health disparities. JAMA. 2009;301:2252–2259. doi: 10.1001/jama.2009.754. [DOI] [PubMed] [Google Scholar]
- 9.McEwen B. Protective and damaging effects of stress mediators. N Eng J Med. 1998;338:1–7. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- 10.Gilley D, Herbert B, Huda N, Tanaka H, Reed T. Factors impacting human telomere homeostasis and age-related disease. Mech Ageing Dev. 2008;129:27–34. doi: 10.1016/j.mad.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 11.Fitzpatrick A, Kronmal R, Gardner J, Psaty B, Jenny N, Tracy R, et al. Leukocyte telomere length and cardiovasculardisease in the cardiovascular health study. Am J Epidemiol. 2007;165:14–21. doi: 10.1093/aje/kwj346. [DOI] [PubMed] [Google Scholar]
- 12.Martin-Ruiz C, Dickinson H, Keys B, Rowan E, Kenny R, Von Zglinicki T. Telomere length predicts poststroke mortality, dementia, and cognitive decline. Ann Neurol. 2006;60:174–180. doi: 10.1002/ana.20869. [DOI] [PubMed] [Google Scholar]
- 13.Valdes A, Andrew T, Gardner J, Kimura M, Oelsner E, Cherkas L, et al. Obesity, cigarette smoking, and telomere length in women. Lancet. 2005;366:662–664. doi: 10.1016/S0140-6736(05)66630-5. [DOI] [PubMed] [Google Scholar]
- 14.Derradiji H, Bekaert S, De Meyer T, Jacquet P, Abou-El-Ardat K, Ghardia M, et al. Ionizing radiation-induced gene modulations, cytokine content changes and telomere shortening in mouse fetuses exhibiting forelimb defects. Dev Biol. 2008;322:302–313. doi: 10.1016/j.ydbio.2008.07.032. [DOI] [PubMed] [Google Scholar]
- 15.Bull C, Fenech M. Genome-health nutrigenomics and nutrigenetics: nutritional requirements or ‘nutriomes’ for chromosomal stability and telomere maintenance at the individual level. Proc Nutr Soc. 2008;67:146–156. doi: 10.1017/S0029665108006988. [DOI] [PubMed] [Google Scholar]
- 16.Zglinicki V. Oxidative stress shortens telomeres. Trends Biochem Sci. 2002;27:339–344. doi: 10.1016/s0968-0004(02)02110-2. [DOI] [PubMed] [Google Scholar]
- 17.Epel E, Blackburn E, Lin J, Dhabhar F, Adler N, Morrow J, et al. Accelerated telomere shortening in response to life stress. Proc Nat Acad Sci. 2004;101:17312–17315. doi: 10.1073/pnas.0407162101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tyrka A, Price L, Kao H, Porton B, Marsella S, Carpenter L. Childhood maltreatment and telomere shortening: preliminary support for an effect on early stress on cellular aging. Biol Psychaitry. 2009;67:531–534. doi: 10.1016/j.biopsych.2009.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kananen L, Surakka I, Pirkola S, Suvusaari J, Lonnqvist J, Peltonen L, et al. Childhood adversities are associated with shorter telomere length at adult age both in individuals with an anxiety disorder and controls. PLos ONE. 2010;5:e10826. doi: 10.1371/journal.pone.0010826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Simon N, Smoller J, McNamara K, Maser R, Zalta A, Pollack M, et al. Telomere shortening and mood disorders: prelminary support for chronic stress model of accelerated aging. Biol Psychiatry. 2006;60:432–435. doi: 10.1016/j.biopsych.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 21.Damjanovic A, Yang Y, Glaser R, Keicolt-Glaser J, Nguyen H, Laskowski B, et al. Accelerated telomere erosion is associated with a declining immune function of caregivers of Alzheimer’s disease patients. J Immunol. 2007;179:4249–4254. doi: 10.4049/jimmunol.179.6.4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lung F, Chen N, Shu B. Genetic pathway of major depressive disorder in shortening telomeric length. Psychiatr Genet. 2007;17:195–199. doi: 10.1097/YPG.0b013e32808374f6. [DOI] [PubMed] [Google Scholar]
- 23.Parks C, Miller D, McCanlies E, Cawthorn R, Andrew M, DeRoo LA, et al. Telomere length, current perceived stress, and urinary stress hormones in women. Cancer Epidemiol Biomarkers Prev. 2009;18:551–560. doi: 10.1158/1055-9965.EPI-08-0614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rufer N, Brummendorf T, Kolvraa S, Bischoff C, Christensen K, Wadsworth L, et al. Telomere flouresence measurements in granulocytes and T lymphocyte subsets point to a high turnover of hematopoietic stem cells and memory T cells in early childhood. J Exp Med. 1999;190:157–167. doi: 10.1084/jem.190.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cameron N, Demerath E. Critical periods in human growth and their relationship to diseases of aging. Am J Phys Anthropol. 2002;35:159–184. doi: 10.1002/ajpa.10183. [DOI] [PubMed] [Google Scholar]
- 26.Nelson C, Zeanah C, Fox N, Marshall P, Smyke A, Guthrie D. Cognitive recovery in socially deprived young children: The Bucharest Early Intervention Project. Science. 2007;318:1937–1940. doi: 10.1126/science.1143921. [DOI] [PubMed] [Google Scholar]
- 27.Pollak S, Nelson C, Schlaak M, Roeber B, Wewerka S, Wiik K, et al. Neurodevelopmental effects of early deprivation in post-institutionalized children. Child Dev. 2010;81:224–236. doi: 10.1111/j.1467-8624.2009.01391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sheridan M, Drury S, McLaughlin K, Amas A. Early institutionalization: neurobiological consequences and genetic modifiers. Neuropsychol Rev. 2010;20:414–429. doi: 10.1007/s11065-010-9152-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smyke A, Zeanah C, Fox N, Nelson C, Guthrie D. Placement in foster care enhances attachment among young children in institutions. Child Dev. 2010;81:212–223. doi: 10.1111/j.1467-8624.2009.01390.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Drury S, Theall K, Smyke A, Keats B, Egger H, Nelson C, et al. Modification of depression by the COMT val 158met polymorphisms in children exposed to early social deprivation. Child Abuse Negl. 2010;34:387–395. doi: 10.1016/j.chiabu.2009.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kumsta R, Stevens S, Brookes K, Schlotz W, Castle J, Beckett C, et al. 5HTT genotype moderates the influence of early institutional deprivation on emotional problems in adolescence: evidence from and English and Romanian Adoptee (ERA) study. J Child Psychol Psychiatry. 2010;51:755–762. doi: 10.1111/j.1469-7610.2010.02249.x. [DOI] [PubMed] [Google Scholar]
- 32.Matthews K, Stoney C. Influences of sex and age on cardiovascular responses during stress. Psychosom Med. 1988;50:46–56. doi: 10.1097/00006842-198801000-00006. [DOI] [PubMed] [Google Scholar]
- 33.Kessler R, Brown R, Broman C. Sex differences in psychiatric help-seeking: Evidence from four large-scale surveys. J Health Soc Behav. 1982;22:49–64. [PubMed] [Google Scholar]
- 34.Kessler R, McLeod J. Sex differences in vulnerability to undesirable life events. Am Sociol Rev. 1984;49:620–631. [Google Scholar]
- 35.Bebbington P, Dunn G, Jenkins R, Lewis G, Brugha T, Farrell M, et al. The influence of age and sex on the prevalence of depressive conditions: report from the National Survey of Psychiatric Morbidity. Int Rev Psychiatry. 2003;15:74–83. doi: 10.1080/0954026021000045976. [DOI] [PubMed] [Google Scholar]
- 36.Barnett J, Heron J, Ring S, Goldring J, Goldman D, Xu K, et al. Gender-specific effects of the catechol-o-methyltransferase val 108/158 met polymorphism on cognitive function in children. Am J Psychiatry. 2007;164:142–149. doi: 10.1176/ajp.2007.164.1.142. [DOI] [PubMed] [Google Scholar]
- 37.Zeanah C, Nelson C, Fox N, Smyke A, Marshall P, Parker S, et al. Designing research to study the effects of institutionalization on brain and behavioral development: The Bucharest Early Intervention Project. Dev Psychopathol. 2003;15:885–907. doi: 10.1017/s0954579403000452. [DOI] [PubMed] [Google Scholar]
- 38.Zeanah C, Egger H, Smyke A, Nelson C, Fox N, Marshall P, et al. Institutional rearing and psychiatric disorders in Romanian pre-school children. Am J Psychiatry. 2009;166:777–785. doi: 10.1176/appi.ajp.2009.08091438. [DOI] [PubMed] [Google Scholar]
- 39.Zeanah C, Koga S, Simion B, Stanescu A, Tabacaru C, Fox N, et al. Ethical dimensions of the BEIP: Response to commentary. Infant Ment Health J. 2006;27:581–583. doi: 10.1002/imhj.20117. [DOI] [PubMed] [Google Scholar]
- 40.Smyke A, Koga S, Johnson D, Fox N, Marshall P, Nelson C, et al. The caregiving context in institution reared and family reared infants and toddlers in Romania. J Child Psychol Psychiatry. 2007;48:210–218. doi: 10.1111/j.1469-7610.2006.01694.x. [DOI] [PubMed] [Google Scholar]
- 41.Rufer N, Brummendorf T, Kolvraa S, Bischoff C, Christensen K, Wadsworth L, et al. Telomere flourescence measurements in granulocytes and T-lymphocyte subsets point to a high turnover of hematopoietic stem cells and memory t cells in early childhood. J Exp Med. 1999;190:157–167. doi: 10.1084/jem.190.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Frenck R, Blackburn E, Shannon K. The rate of telomere sequence loss in human leukocytes varies with age. PNAS. 1998;95:5607–5610. doi: 10.1073/pnas.95.10.5607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Prescott J, McGrath M, Lee I, Buring J, De Vivo I. Telomere length and genetic analyses in population-based studies of endometrial cancer risk. Cancer. 2002;116:4275–4282. doi: 10.1002/cncr.25328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tyrka A, Price L, Kao H, Porton B, Marsella S, Carpenter L. Childhood maltreatment and telomere shortening: preliminary support for and effect of early stress on cellular aging. Biol Psychaitry. 2009;67:531–534. doi: 10.1016/j.biopsych.2009.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Beach SRH, Brody G, Todorov A, Gunter T, Philibert R. Methylation at SLC6A4 is linked to a family history of child abuse: An examination of the Iowa adoptee sample. Am J Med Genet B Neuropsychiatr Genet. 2010;153B:710–713. doi: 10.1002/ajmg.b.31028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kinnally EL, Capitanio JP, Leibel R, Deng L, LeDuc C, Haghighi F, et al. Epigenetic regulation of serotonin transporter expression and behavior in infant rhesus macaques. Genes, Brain Behav. 2010;9:575–582. doi: 10.1111/j.1601-183X.2010.00588.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weaver ICG. Shaping adult phenotypes through early life environments. Birth Defects Res C: Embryo Today: Rev. 2009;87:314–326. doi: 10.1002/bdrc.20164. [DOI] [PubMed] [Google Scholar]
- 48.Roth T, Lubin F, Funk A, Sweatt J. Lasting epigenetic influence of early-life adversity on BDNF gene. Biol Psychaitry. 2009;65:760–769. doi: 10.1016/j.biopsych.2008.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Oberlander T, Weinberg J, Papsdorf M, Grunau R, Misri S, Devlin A. Prenatal exposure to maternal depression, neonatal methylation of human glucocorticoid receptor gene (NR3C1) and infant cortisol stress responses. Epigenetics. 2008;3:97–106. doi: 10.4161/epi.3.2.6034. [DOI] [PubMed] [Google Scholar]
- 50.McGowan P, Sasaki A, D’Alessio A, Dymov S, Labonté B, Szyf M, et al. Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nat Neurosci. 2009;12:342–348. doi: 10.1038/nn.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dolinoy D, Weidman J, Jirtle R. Epigenetic gene regulation: linking early developmental environment to adult disease. Reprod Toxicol. 2007;23:297–307. doi: 10.1016/j.reprotox.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 52.Wong C, Caspi A, Williams B, Craig I, Houts R, Ambler A, et al. A longitudinal study of epigenetic variation in twins. Epigenetics. 2010;5:1–11. doi: 10.4161/epi.5.6.12226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McGowan P, Suderman M, Sasaki A, Huang T, Hallet M, Meaney MJ, et al. Broad epigenetic signature of maternal care in the brain of adult rats. PLos ONE. 2011;6:e14739. doi: 10.1371/journal.pone.0014739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Matthews K, Stoney C. Influences of sex and age on cardiovascular responses during stress. Psychosom Med. 1988;50:46. doi: 10.1097/00006842-198801000-00006. [DOI] [PubMed] [Google Scholar]
- 55.Magwire M, Yamamoto A, Carbone M, Roshina N, Symonenko A, Pasyukova E, et al. Quantitative and molecular genetic analyses of mutations increasing Drosophila life span. Plos Genet. 2010;6:e1001037. doi: 10.1371/journal.pgen.1001037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Johnson D, Guthrie D, Smyke A, Koga S, Fox N, Zeanah C, et al. Growth and associations between auxology, caregiving environment, and cognition in social deprived Romanian children randomized to foster vs ongoing institutional care. Arch Pediatr Adolesc Med. 2010;164:507–516. doi: 10.1001/archpediatrics.2010.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thomas P, O’Callaghan N, Fenech M. Telomere length in white blood cells, buccal cells and brain tissue and its variation with ageing in Alzheimer’s disease. Mech Ageing Dev. 2008;129:183–190. doi: 10.1016/j.mad.2007.12.004. [DOI] [PubMed] [Google Scholar]