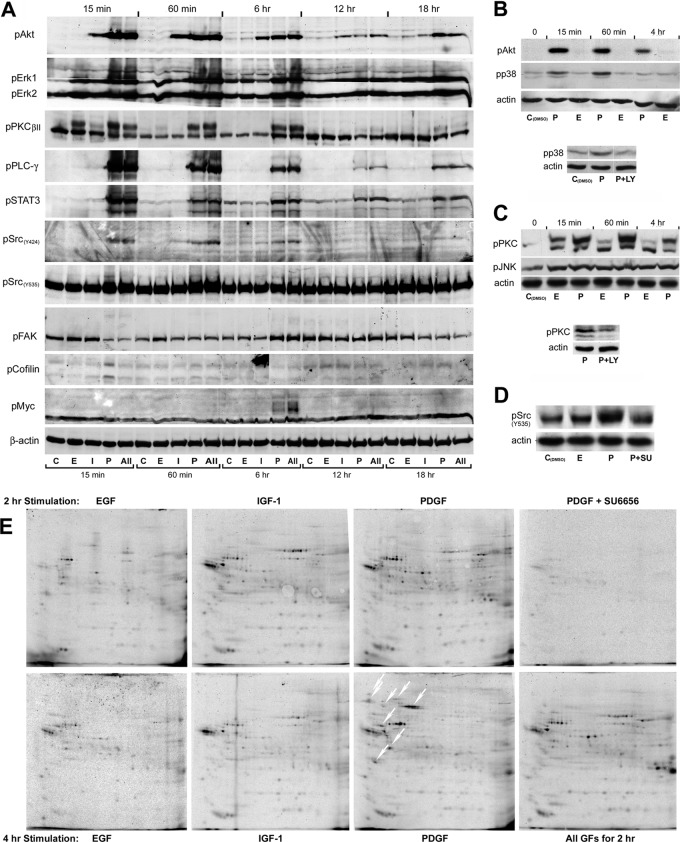
Fig. 3.
Analysis of growth factor induced phospho-signaling. A, Temporal changes in key signal transducer activities demonstrated by immunoblotting with phosphorylation site-specific antibodies. β-actin served as loading control. B, Immunoblot analysis of Akt and p38 phosphorylation in PDGF and EGF treated cells (top). Pretreatment with the PI3K-inhibitor LY294002 (10 μm) abolished the PDGF-induced increase in p38 phosphorylation at the 60 min time point (bottom). C, Immunoblot analysis of PKC and JNK phosphorylation in EGF and PDGF treated cells. Both GFs induced a weak, long-lasting increase in JNK phosphorylation. Although a high level of phospho-activated PKC was sustained for several hours in PDGF treated cells, less than 30% of the increase in PKC phosphorylation found in EGF treated cells at the 15 min time point were detected after 60 min treatment. Pretreatment with LY294002 reduced the level of phosphorylated PKC in cells treated with PDGF for 60 min (bottom). D, The PDGF-induced increase in phosphorylation of c-Src at the inhibitory site (Y535) at the 15 min time point was abolished by pretreatment with the Src-specific inhibitor SU6656 (2 μm), indicating that the modification is induced by autophosphorylation. E, two-dimensional autoradiograms of [33P]-orthophosphate labeled proteins isolated by immunoprecipitation with an anti-c-Src antibody following 2 h and 4 h growth factor treatment. The white oblique downwards arrows indicates phosphoproteins that coprecipitated with Src from cells treated with PDGF for 4 h, but were absent or weakly represented in immunoprecipitates from cells that had been exposed to the growth factor for 2 h. The radiolabeled phosphoprotein isolates were separated by IPHGE/SDS-PAGE, employing broad pH-range (3–10 NL) dry strips in the first dimension.