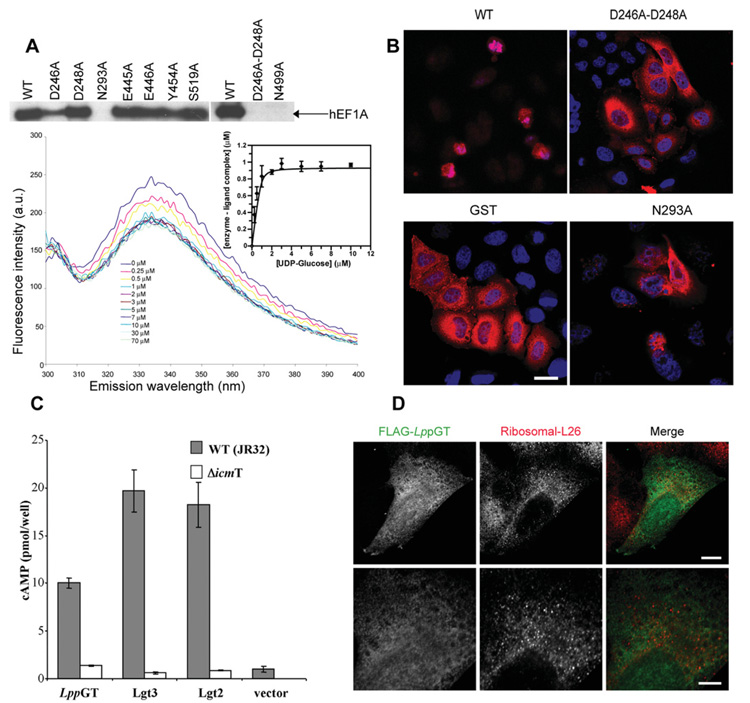
Figure 5. Site-directed mutagenesis, microinjection, translocation and localization studies.
(A) The upper panel shows autoradiography of wild-type and mutant enzymes incubated with HEK-293 lysates and UDP-[3H]glucose. The lower panel shows quenching of intrinsic LppGT tryptophan fluorescence measured at increasing concentrations of UDP-glucose. All data points represent the means ± S.D. for three measurements. The Kd for UDP-glucose was determined by fitting fluorescence intensity data against free UDP-glucose concentration (insert). See Table 2 for the Kd values for wild-type and mutant enzymes. (B) HeLa cells microinjected with wild-type LppGT, D246A/D248A double-mutant LppGT (D246A-D248A), LppGT-N293A and GST, as a control, at a protein concentration of 30 μM in the injection needle. Cells were co-injected with Texas Red-conjugated dextran and incubated for 48 h and are counterstained with DAPI. Representative images are shown for the cells after 48 h incubation. After injection of wild-type LppGT, there were few surviving red (Texas-Red–dextran-postive) cells and, of these, most had a rounded-up morphology when compared with cells injected with GST protein and the double mutant LppGT. LppGT-N293A was slightly protective compared with the wild-type enzyme. (C) Translocation experiments. All three enzymes were translocated through the Icm/Dot machinery (grey bars) compared with a mutated ΔicmT Legionella strain (white bars) as measured by cAMP concentrations. (D) Microinjected FLAG-tagged LppGT protein was injected into HeLa cells and incubated for 24 h. The FLAG-tagged protein (green) was diffusely distributed throughout the cell and showed rare co-localization with the counterstain against ribosomal L26 protein (red). The lower panels show a higher magnification of the image in the upper panels. Scale bar, 10 μm (upper panels) or 5 μm (lower panels). The proteins are FLAG-tagged at the C-terminus.