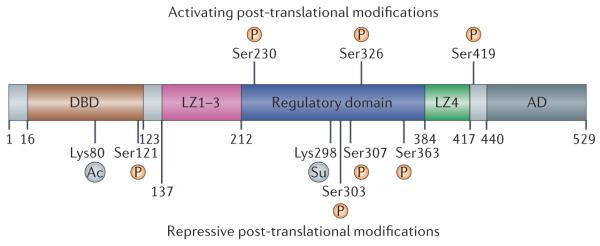
Figure 3. HSF1 regulation by post-translational modifications.
In response to cellular stress, heat shock transcription factor 1 (HSF1) is hyperphosphorylated on up to 12 serine residues; this occurs in parallel with HSF1-dependent transactivation. Three sites of phosphorylation involved in HSF1 activation that have been studied in detail are shown. HSF1 activity is also repressed by constitutive phosphorylation of Ser303 and Ser307, and by stress-responsive sumoylation of Lys298 (represented on the figure as ‘Su’). The binding of HSF1 to DNA is inhibited by the acetylation of Lys80 (represented on the figure as ‘Ac’). AD, activation domain; DBD, DNA binding domain; LZ1, leucine zipper domain 1.