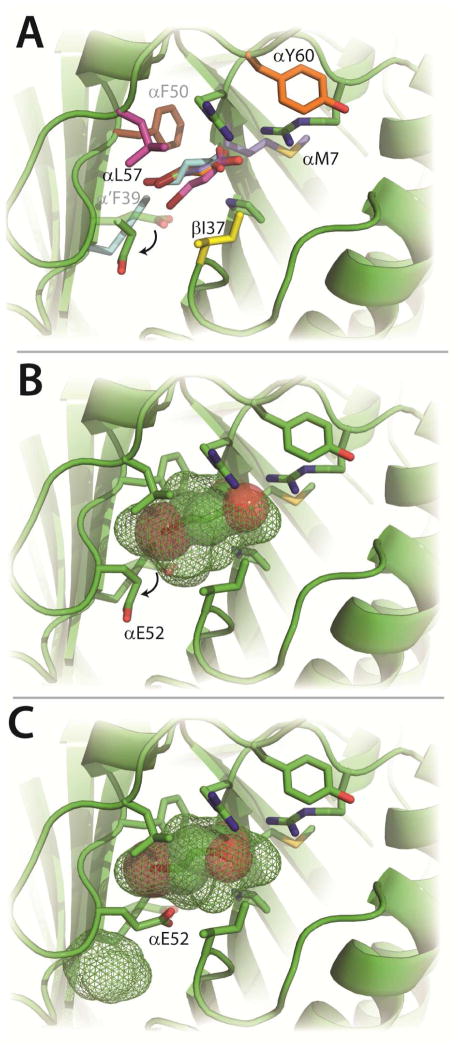
Figure 2. Substrate Docking Studies.
A) Six docking poses of 9 in the CaaD active site having the lowest energies of binding (ΔG values can be found in Table S1) after each residue (shown in color) was independently replaced with a tryptophan (in silico). Four poses of 9 are superimposable, whereas the poses of 9 in the α’F39W (cyan) and αL57W (pink) enzymes are slightly different. All poses rotate E52 as illustrated by the arrow. B) Docking of 9 (spheres) into wild-type CaaD.19 Shown in mesh is the in silico active site pocket volume. The movement of αE52 to expand the active site volume is shown by the arrow. C) An alternative docking mode for 9 with αE52 in a position to perform the proposed chemistry with the newly predicted active site volume shown in mesh. The figure was prepared using PyMOL.20