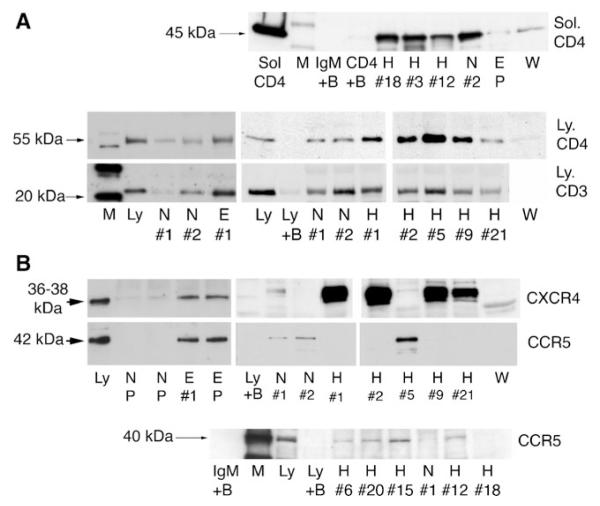
Fig. 2.
Immunoprecipitation experiments to show binding of IgM to CD3, CD4, CCR5, and CXCR4 obtained from leukocytes. In panels a and b, identical quantities of individual (labeled #1, #2, etc.) or pooled (P) IgM from normal (N), HIV (H), ESRD (E), or Waldenstrom (W) were used to immunoprecipitate leukocyte receptors from equal amounts of whole cell lysates. As controls, Western blots were performed with cell lysates in the absence of agarose beads (to control for binding of primary antibody to leukocyte receptor and to determine receptor size (labeled Ly). In another control, agarose beads without IgM were added to lysate to determine whether the leukocyte receptor nonspecifically bound to the bead (B plus Ly). Note that several fold more receptors were immunoprecipitated by ESRD and HIV IgM when compared with normal IgM. In a, recombinant, glycosylated, solubilized CD4 (Sol-CD4), comprising the full-length extracellular domain was immunoprecipitated by IgM. For controls, Western blots were performed with soluble CD4 in absence of IgM and agarose beads (labeled CD4) and with Sol-CD4 added to agarose beads in absence of IgM (labeled CD4+ B). Lobo PI, Schlegel KH, Spencer CE, Okusa MD, Chisholm C, Mchedlishvili N, et al.: Naturally occurring IgM anti-leukocyte autoantibodies (IgM-ALA) inhibit T cell activation and chemotaxis. J. Immunol. 180:1780-1791, 2008. Copyright 2008. The American Association of Immunologists, Inc