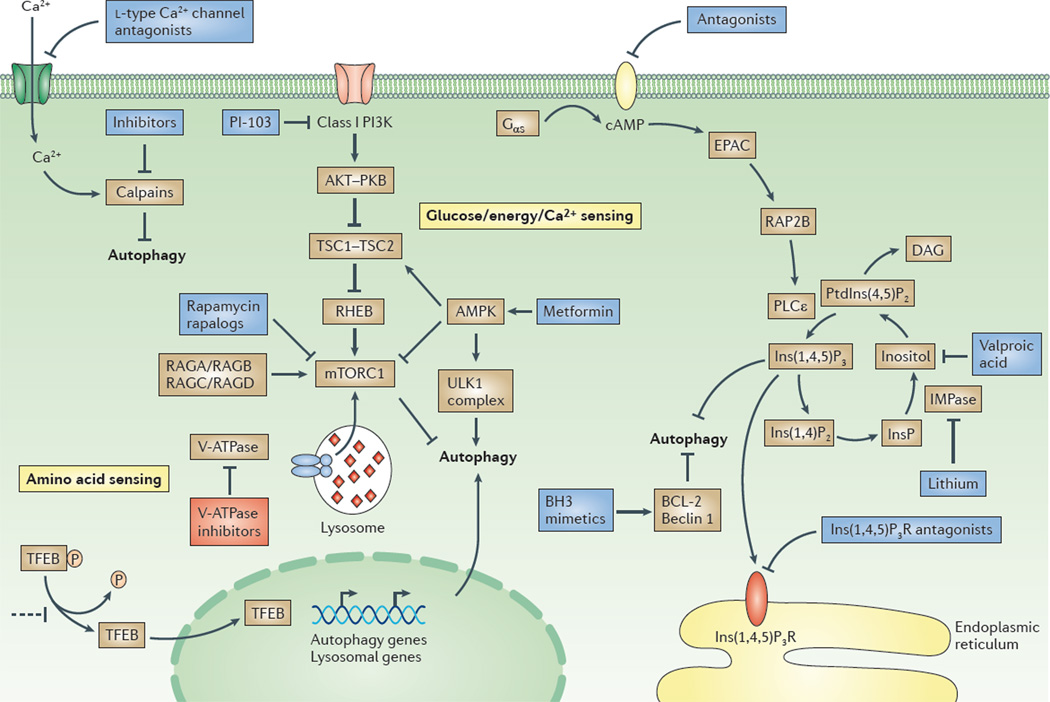
Figure 1. Overview of the regulation of macroautophagy and potential drug targets.
Two major signalling pathways are depicted here: the pathway involving class I phosphoinositide 3-kinase (PI3K), protein kinase B (PKB) and mammalian target of rapamycin complex 1 (mTORC1), and a cyclical mTOR-independent pathway; the basic helix–loop–helix leucine zipper transcription factor EB (TFEB)-mediated pathway is also depicted213. TFEB regulates the expression of the genes involved in the different stages of autophagy between autophagosome formation and cargo degradation (see FIG. 2). In nutrient-rich medium, TFEB is phosphorylated by mTORC1 and is retained in the cytoplasm. In starved cells, TFEB is dephosphorylated and is translocated into the nucleus. TFEB is a potential target for drugs. Retaining TFEB in the cytoplasm would inhibit autophagy, as illustrated in the figure. By contrast, promoting the nuclear translocation of TFEB would stimulate autophagy. Activating the class I PI3K–PKB–mTORC1 pathway by growth factors and amino acids blocks autophagy by inhibiting the initiation of autophagosome formation by the UNC51-like kinase 1 (ULK1) complex. Glucose starvation increases the AMP/ATP ratio, which activates AMP-activated protein kinase (AMPK). This enzyme induces autophagy by inhibiting mTORC1 (via directly targeting elements of the complex) or by phosphorylating and activating tuberous sclerosis 2 (TSC2) in the TSC1–TSC2 complex. Recent data suggest that mTOR interacts with TFEB, as the two proteins are colocalized on lysosomal membranes. Phosphorylation of mTOR inhibits TFEB acivity, whereas ATP-competitive mTOR inhibitors enable TFEB dephosphorylation, thus allowing its nuclear translocation and activation218. AMPK also phosphorylates and thus activates the ULK1 complex. Activators of AMPK (such as metformin) stimulate autophagy, as do inhibitors of mTORC1 (for example, rapamycin or rapalogues) and inhibitors of class I PI3Ks (for example, PI-103). Amino acids activate mTORC1 via RAG GTPases, and also via the vacuolar V-ATPase located in the lysosomal membrane219. Inhibitors of V-ATPases, such as bafilomycin A1, block the maturation of autophagosomes. However, blocking V-ATPase also inhibits the amino-acid-dependent activation of mTORC1, which would have a stimulatory effect on autophagosome formation. Elevation of the intracellular levels of cyclic AMP (cAMP) by adenylyl cyclase downstream of G protein-coupled receptors blocks autophagy by activating the exchange protein directly activated by cAMP (EPAC), the small G protein RAP2B and phospholipase Cε (PLCε). Activation of PLCε results in the production of inositol-1,4,5-trisphosphate (Ins(1,4,5)P3) and, consequently, the release of Ca2+ from the endoplasmic reticulum via the Ins(1,4,5)P3 receptor (Ins(1,4,5)P3R). Influx of Ca2+ into the cytoplasm is also triggered by l-type Ca2+ channel agonists. Drugs acting at the various different steps of the cAMP–EPAC–PLCε– Ins(1,4,5)P3 pathway regulate autophagy. Calpains activated by Ca2+ block autophagy by cleaving and constitutively activating Gsα proteins (also called Gαs), which increases cAMP levels. Thus, inhibitors of calpains would stimulate autophagy in this setting. The binding of B cell lymphoma 2 (BCL-2) to beclin 1 inhibits autophagy, and this effect can be counteracted by BCL-2 homology 3 (BH3) mimetics. DAG, diacylglycerol; IMPase, inositol monophosphatase; InsP, inositol monophosphate; Ins(1,4)P2, inositol-1,4-bisphosphate; PtdIns(4,5)P2, phosphatidylinositol-4,5-bisphosphate; RHEB, RAS homologue enriched in brain (GTP binding protein).