Fig. 7.3. Evolution of co-complex interactions in the group II chaperonins.
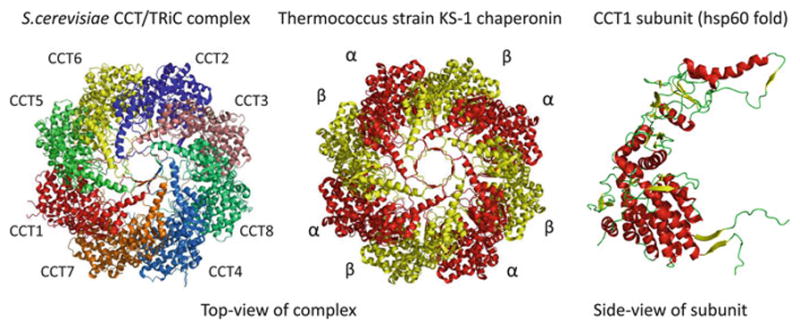
Computational studies have shown that protein complexes usually evolve by duplication and divergence of their subunits. The group II chaperonin complexes provide a good illustration of this general trend. The archeal group II chaperonin complexes (termed thermosomes) usually contain 1–3 homologous chaperonins and it represented here by the thermosome of Thermococcus strain KS-1 (PDB:1Q2V). The eukaryotic complexes (called TriC or CCT) are composed of eight chaperonin paralogs, represented here by the S. cerevisiae CCT complex (PDB:3P9E). All of the subunits are structurally similar, exemplified here by the S. cerevisiae CCT1 subunit structure
