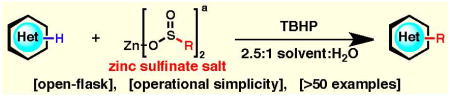
Table 1. Substrate scope of the zinc sulfinate salt toolkit.
Isolated yields are displayed, % conversions by GC-MS are indicated between parentheses, and regioisomeric ratios are shown between square brackets. Compounds 1A, 2A, 3A, 4A and 5A have been previously synthesized with Langlois’ reagent (ref. 7); 1B, 2B, 3B, 4B, 5B and 8B have been previously prepared using DFMS (ref. 9) and are included herein for completeness; all other compounds in this table are new.
| ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| Zn salt; R =
|
CF3 (A) | CF2H (B) | CF2CF3 (C) | CF2F (D) | CH(CH3)2 (E) | (CH2CH2O)3CH3 (F) |
| heterocycle | ||||||
|
| ||||||
1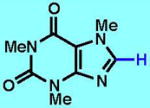
|
89 (100)b | 73 (57)bk | 51g | 80g | 41h | 40i |
| 1A | 1B | 1C | 1D | 1E | 1F | |
|
| ||||||
2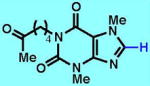
|
79 (100)b | 72 (41)bk | 44g | 75g | 37h | 49i |
| 2A | 2B | 2C | 2D | 2E | 2F | |
|
| ||||||
3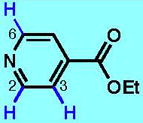
|
35 (77)b [4:1 C2:C3] |
66 (100)b | 18 (85)g [4:1 C2:C3] |
73fj [17:1 C2:C2&C6] |
47d | 41i |
| 3A | 3B | 3C | 3D | 3E | 3F | |
|
| ||||||
4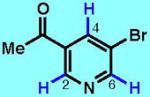
|
66 (65)bk [2.3:1 C6:C2] |
60 (96)b | 33g [1.4:1 C6:C4] |
N.R. | 41i | N.R. |
| 4A | 4B | 4C | 4D | 4E | 4F | |
|
| ||||||
5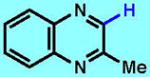
|
75 (100)b [5 products] |
50 (67)b | 31 (77)c | 56g | 43h | 32h |
| 5A | 5B | 5C | 5D | 5E | 5F | |
|
| ||||||
6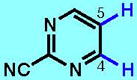
|
42 (44)e [2.7:1 C4:C5] |
21 (44)e [1.6:1 C4:C5] |
21h | N.R. | 46h [2.1:1 C4:C5] |
16i [3.4:1 C4:C5] |
| 6A | 6B | 6C | 6D | 6E | 6F | |
|
| ||||||
7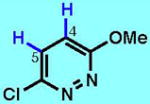
|
45 (90)e | 57 (71)e [6:1 C4:C5] | N.R. | N.R. | 49h [10:1 C4:C5] |
32 (38)i |
| 7A | 7B | 7C | 7D | 7E | 7F | |
|
| ||||||
8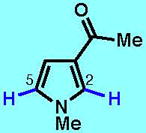
|
76 (91)b [7.4:1 C2:C5] |
65 (100)b | 58h [1.4:1 C2:C5] |
40d | 17h | 10 (43)i |
| 8A | 8B | 8C | 8D | 8E | 8F | |
Standard conditions involve heterocycle (1.0 equiv), zinc salt (2.0–3.0 equiv), TBHP (3.0–5.0 equiv) and solvent:H2O (2.5:1) at a specified temperature for a period of 3–12 h. Solvent/temperature:
CH2Cl2, RT;
ClCH2CH2Cl, RT;
ClCH2CH2Cl, 50 °C;
perfluorohexane, RT;
perfluorotoluene, RT;
perfluorotoluene, 50 °C;
DMSO, 50 °C;
anisole, 50 °C (note: the reaction time for anisole is 0.5–96 h, see Supplementary Information).
TFA was used as an additive.
When the GC % conversion is lower than the isolated yield, it signifies that only one addition of Zn salt was made for the “GC yield reaction”, but that a second addition of Zn salt and TBHP was performed after 12 h for the “isolated yield reaction”.
TBHP = tert-butyl hydroperoxide; TFA = trifluoroacetic acid; RT = room temperature; N.R. = no reaction.
