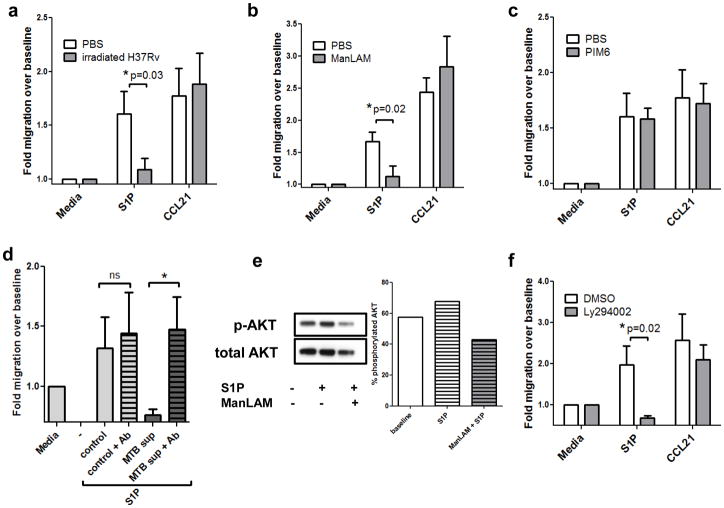
Figure 3. Migratory responses of mouse T lymphocytes after exposure to tuberculosis antigens.
Mouse T cells were pre-incubated with (A) 1mg/mL whole irradiated H37Rv, (B) 100ng/mL ManLAM or (C) 100ng/mL PIM6 for 2 hours. Cells were washed and placed in Transwell chambers and stimulated for migration induced by media control, S1P (10nM), or CCL21 (50ng/ml). The assay was terminated after 3.5 hours, and migration expressed as fold change over baseline (media control expressed as 1). Data in panels A–C are from three independent experiments performed in triplicate (mean ± s.e.m., *P=0.03 and *P=0.02, Student’s t test). (D) Culture supernatants from control or MTB-infected mouse bone marrow-derived macrophages were harvested 48h post-infection. Supernatants were precleared with CS35 anti-ManLAM antibodies at 4°C overnight. Primary mouse T cells were pretreated with the supernatants for 1.5h prior to Transwell assay. The data depicted shows migration over baseline to S1P at 10nM. (Duplicate wells from 2 separate experiments; mean ± s.e.m.,*P<0.05, Student’s t test). (E) Western blotting analysis of mouse T cells pretreated with ManLAM or PBS for 2h, then stimulated with S1P or vehicle for 5 minutes. Percent phosphorylated AKT was determined by creating a ratio of densitometry of phospho-AKT Ser473 to total AKT. (Representative blot, n=2 separate experiments). (F) Mouse T lymphocytes were pretreated with 50μM of the PI3K/AKT inhibitor Ly294002 for 1h prior to Transwell migration. S1P migration was significantly inhibited, whereas migration to CCL21 was not significantly affected (n=3 experiments performed in triplicate,*P=0.02, Student’s t test).