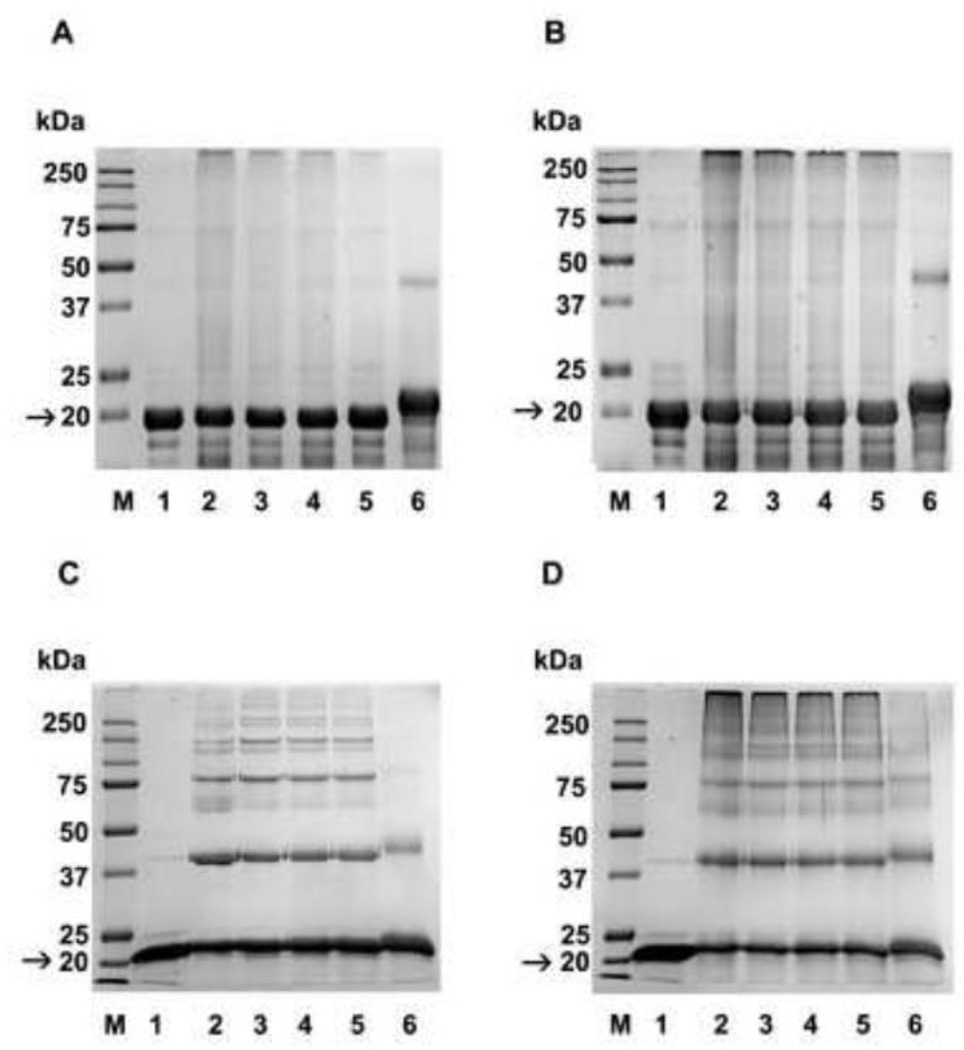
Fig. 3. Nε-acetylation of lysine residues decreases AGE-mediated crosslinking in α-crystallin.
Human αA- and αB-crystallin were acetylated with 0.05, 0.5, 1, and 10 fold molar excesses (relative to lysine) of Ac2O. Unacetylated and acetylated αA and αB-crystallin were then glycated with MGO or ascorbate and subjected to SDS-PAGE analysis. A, αA-crystallin glycated with MGO; B, αA-crystallin glycated with ascorbate; C, αB-crystallin glycated with MGO; D, αB-crystallin glycated with ascorbate. In all panels, lane 1=unacetylated protein, lane 2=glycated protein, lanes 3 to 6 are glycated and acetylated, respectively, with 0.05, 0.5, 1, and 10 fold molar excess Ac2O. M=molecular weight markers. Arrow indicates αA or αB-crystallin in samples.