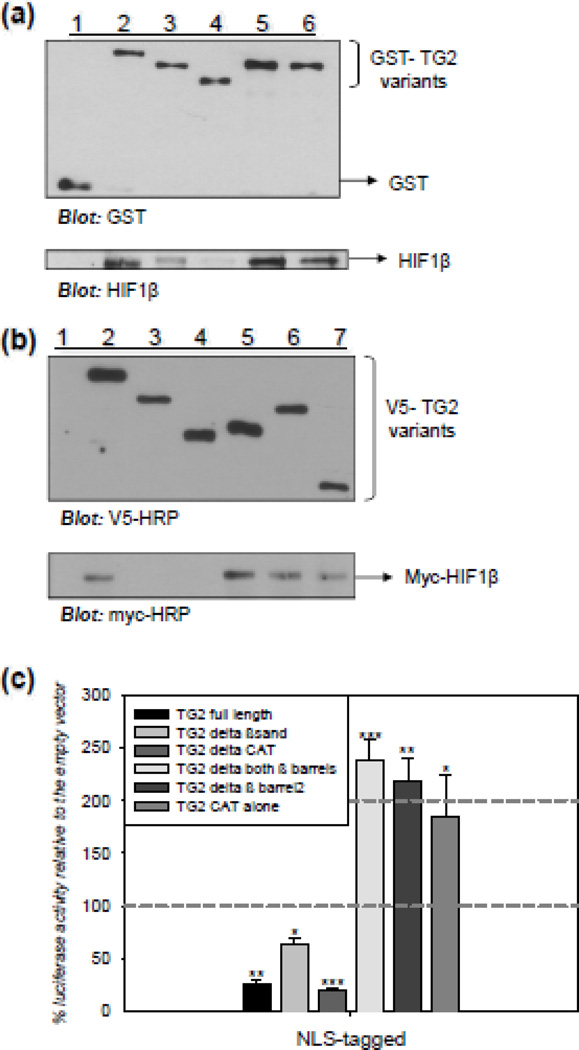
Figure 5. Interacting with HIF1β is not required for TG2 to suppress HIF-dependent transcription.
(a) TG2 and HIF1β interact in vitro through the catalytic core domain of TG2. Representative immunoblot from a GST pull-down assay with GST-TG2 variants as bait and HIF1β as prey. Immunoblot analysis for HIF1β confirms that HIF1β was pulled down by full length GST-TG2 (lane 2), GST-TG2Δβ-barrel1 (lane 5), and GST-TG2Δβ-barrel2 (lane 6); but not by GST alone (lane 1) and GST-TG2ΔCAT (lane 4). HIF1β was pulled down by GST-TG2Δβ-sandwich to a lesser extent (lane 3). (b) TG2 and HIF1β interact through the catalytic core domain of TG2 in human cells. Representative immunoblot from a co-immunoprecipitation assay with V5-TG2 variants as bait and myc-HIF1β as prey. HEK-293A cells were transfected with V5-tagged TG2 deletion constructs and the myc-HIF1β construct. The cells were lysed after 48 h. Immunoblot analysis for myc confirms that HIF1β interacts with full length V5-TG2 (lane 2), V5-TG2Δboth-β-barrels (lane 5), V5-TG2Δβ-barrel2 (lane 6), and V5-TG2-CAT alone (lane 7); but not by V5 tag alone (lane 1), V5-TG2Δβ-sandwich (lane 3), and V5-TG2ΔCAT (lane 4). (c) Interacting with HIF1β is not required for TG2 to suppress HIF-dependent transcription. Relative HRE luciferase activity in MCF-7 cells transiently transfected with NLS-TG2 constructs after 15 h of 0.1% oxygen treatment in the presence of 2.5 µM NC9. Data is presented as percent of empty vectors. NLS-tagged TG2 full length, TG2Δβ-sandwich and TG2ΔCAT constructs significantly suppressed HRE activity; however, NLS-tagged TG2Δboth-β-barrels, TG2Δβ-barrel2 and TG2-CAT alone constructs significantly induced HRE activity (N=4). Results are shown as mean ± SEM *p<0.05, **p<0.01, ***p<0.005.