It has been practically 20 years since polymorphisms in the apolipoprotein E gene (APOE) were first linked to the risk for developing Alzheimer's disease. And like so many other aspects of this disorder, the mechanistic explanation for this link remains a mystery. One thing is clear, however: individuals carrying an APOE ε4 allele have a higher rate of Alzheimer's disease and greater accumulations of amyloid β-peptide (Aβ), the molecular species increasingly indicted as the key perpetrator of Alzheimer pathogenesis. However, evidence indicates that APOE genotype may alter neurophysiology and biochemistry independently of Aβ production or accumulation. Differences in neurological activity detected by fMRI and fluorodeoxyglucose (fDG)-PET are associated with possession of an APOE ε4 allele in undemented older subjects and in those too young to have significant Aβ accumulation (Bookheimer and Burggren 2009). ApoE protein has been proposed to play important roles in brain function via its impact on cerebral atherosclerosis, interstitial cholesterol distribution, cytoskeletal structure, and even immunology. But some of the most intriguing effects of APOE genotypic variation relate to synaptic activity, where ApoE variants may differentially modulate glutamate receptors and other synaptic components via interactions with their signal transducing receptors apoER2 and VLDLR. These receptors also act as receptors for reelin, which has well established effects on NMDA receptor activity and long-term potentiation (Reddy et al. 2011).
In this issue, Dumanis et al. (2012) report on their analysis of presynaptic factors in mice genetically modified by replacement of their APOE gene with the polymorphic human sequences. A key finding was that mice expressing the ε4 version of the human ApoE gene had lower levels of phosphate-activated glutaminase, an enzyme that resides in mitochondria to provide Glu as an intermediate energy source for oxidative phosphorylation. Glutaminase has also been credited with a specialized role in the CNS: Glutamatergic neurons have long been considered deficient in the de novo synthesis of Glu. The Glu they release into the synaptic cleft is rapidly cleared by astrocytes. Glutamine synthetase in the astrocytes converts the Glu to Gln, which is then resupplied to the presynaptic bouton. There, glutaminase is generally thought to be critical for converting the amino acid back to Glu for loading into synaptic vesicles as a neurotransmitter. Consistent with this idea, Dumanis et al. found that ApoE4-targeted replacement mice had lower total Glu levels at ages 4-5 months and elevated Gln levels that persisted throughout the first year of life. These findings suggest a presynaptic disturbance that could contribute to the neurophysiological effects of APOE genotype.
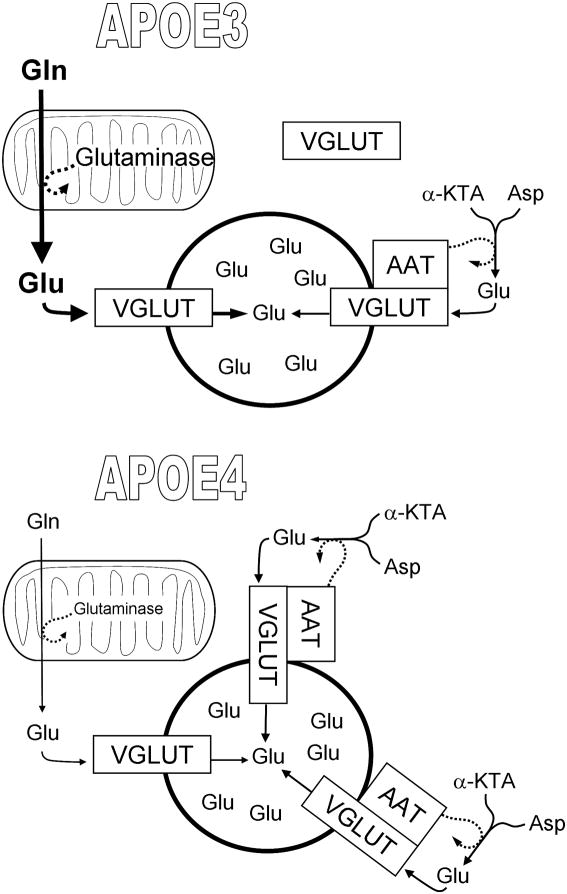
Despite their deficiency at the earliest ages analyzed, Glu levels had normalized by the one-year mark in the ApoE4 mice. Intriguingly, Dumanis et al. also confirmed an increase in the levels of a Glu transporter that participates in loading Glu into synaptic vesicles; as reported previously (Kariv-Inbal et al. 2012), VGLUT1 was elevated in ApoE4-expressing mice. Together with another recent Journal of Neurochemistry article (Takeda et al. 2012), these findings suggest a novel scenario. Takeda et al. reported evidence that a previously unrecognized capacity for Glu synthesis resides in the synaptic vesicles themselves, utilizing α-ketoglutarate (α-KGA) as a starting block and aspartate as an amine-group donor. The interesting connection of this vesicular aspartate aminotransferase (AAT) to Dumanis et al. is the evidence that the vesicular AAT is coupled in some way to the activity of VGLUT. This may indicate a process by which Glu levels in the older ApoE4-mice were normalized and synaptic vesicles could be loaded with an equivalent quantal cargo. Note also that Dumanis et al. report a good fraction of VGLUT1 to be cytosolic in ApoE3 mice, whereas the transporter was completely restricted to the membrane fraction in ApoE4 mice. Takeda et al. found the vesicular AAT to be bound ionically to the cytosolic leaflet of the vesicular membrane, suggesting that its association with the vesicles may be variable and/or regulated.
The question then becomes Where is the impact of ApoE4 that creates both a diminution of glutaminase and an elevation of VGLUT1? Dumanis et al. present a model involving an initial site of action for ApoE at the presynaptic element. But ApoE receptors are much more abundant in the postsynaptic than the presynaptic compartment (May et al. 2004; Dumanis et al. 2011). It is possible that ApoE actions on presynaptic elements ensue via an indirect mechanism, resulting from an initial effect elsewhere. To wit, agents that directly inhibit NMDA-receptors can diminish the expression of glutaminase, and NMDA itself can elevate it (Moran et al. 1999). More to the point, the diminution in phosphate-activated glutaminase and compensatory increase in VGLUT1 may reflect a shift from reliance on Gln to greater utilization of α-KGA and aspartate for the supply of Glu to synaptic vesicles. This could be the result of an inadequate supply of Gln from astrocytes to the presynaptic terminals in the ApoE4-expressing brain. While there are several elements in this supply cycle that could be impinged upon (Fig. 1), the overall level of Gln does not appear to be the problem; total Gln was elevated in ApoE4 mice at all ages examined. Nevertheless, it cannot be ignored that astrocytes are a key component of brain ApoE biology; their expression of ApoE4 may somehow alter expression of system-N/L transporters or other elements of astrocyte→neuron Gln delivery.
Figure 1. Potential alterations in glutamate delivery to synaptic vesicles in the presence of ApoE4.
A portion of the Glu packaged into synaptic vesicles is produced by mitochondrial glutaminase using Gln supplied from astrocytes. But this supply may be limited in ApoE4-expressing astrocytes. This would hypothetically augment the reliance on an additional source of glutamate in the form of an AAT associated with VGLUT on synaptic vesicles. ApoE4-expressing mice show a diminution of glutaminase levels and an increase in VGLUT1, perhaps connected to a relocalization of the transporter from cytosol to vesicle membranes.
Irrespective of ApoE's initial site of action, presynaptic Aβ precursor protein (APP) is poised to respond to alterations in synaptic-vesicle cycling in an Alzheimer-relevant fashion. APP is present in a subset of synaptic vesicles, and evoked increases in the fusion and reinternalization of synaptic-vesicle membrane appear to result in enhanced cleavage of this vesicle-associated APP (Groemer et al. 2011). It will be of interest to determine what impact the ApoE-associated alterations in glutaminase, VGLUT1, and—potentially—vesicular AAT have on vesicle recycling rates and, by extension, presynaptic production and release of Aβ. If the substitution of α-KGA for Gln as a Glu source in ApoE4 mice is confirmed, it will also be important to determine the metabolic costs of redirecting this member of the TCA cycle.
Acknowledgments
Research in the author's laboratory is supported by grants P20 RR-16460 from the National Center for Research Resources and P01AG012411 from the National Institute on Aging. The author receives royalties from the sales of APP by Sigma-Aldrich Co. LLC.
Abbreviations used
- Aβ
amyloid β-peptide
- ApoE
apolipoprotein E
- apoER2
apolipoprotein receptor 2
- APP
Aβ precursor protein
- CBF
cerebral blood flow
- fDG-PET
fluorodeoxyglucose - positron emission tomography
- LRP1
low-density lipoprotein receptor-related protein 1
- VGLUT
vesicular Glu transporter
- VLDLR
very low-density lipoprotein receptor
References
- Bookheimer S, Burggren A. APOE-4 genotype and neurophysiological vulnerability to Alzheimer's and cognitive aging. Annu Rev Clin Psychol. 2009;5:343–362. doi: 10.1146/annurev.clinpsy.032408.153625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumanis SB, Cha HJ, Song JM, Trotter JH, Spitzer M, Lee JY, Weeber EJ, Turner RS, Pak DT, Rebeck GW, Hoe HS. ApoE receptor 2 regulates synapse and dendritic spine formation. PLoS One. 2011;6:e17203. doi: 10.1371/journal.pone.0017203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groemer TW, Thiel CS, Holt M, Riedel D, Hua Y, Huve J, Wilhelm BG, Klingauf J. Amyloid precursor protein is trafficked and secreted via synaptic vesicles. PLoS One. 2011;6:e18754. doi: 10.1371/journal.pone.0018754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kariv-Inbal Z, Yacobson S, Berkecz R, Peter M, Janaky T, Lutjohann D, Broersen LM, Hartmann T, Michaelson DM. The isoform-specific pathological effects of apoE4 in vivo are prevented by a fish oil (DHA) diet and are modified by cholesterol. J Alzheimers Dis. 2012;28:667–683. doi: 10.3233/JAD-2011-111265. [DOI] [PubMed] [Google Scholar]
- May P, Rohlmann A, Bock HH, Zurhove K, Marth JD, Schomburg ED, Noebels JL, Beffert U, Sweatt JD, Weeber EJ, Herz J. Neuronal LRP1 functionally associates with postsynaptic proteins and is required for normal motor function in mice. Mol Cell Biol. 2004;24:8872–8883. doi: 10.1128/MCB.24.20.8872-8883.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran J, Alavez S, Rivera-Gaxiola M, Valencia A, Hurtado S. Effect of NMDA antagonists on the activity of glutaminase and aspartate aminotransferase in the developing rat cerebellum. Int J Dev Neurosci. 1999;17:57–65. doi: 10.1016/s0736-5748(98)00063-x. [DOI] [PubMed] [Google Scholar]
- Rebeck G William, Dumanis Sonya, DiBattista Amanda, Miessau Matthew, Moussa Charbel. APOE genotype affects the presynaptic compartment of glutamatergic nerve terminals. J Neurochem. 2012 Aug 3; doi: 10.1111/j.1471-4159.2012.07908.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy SS, Connor TE, Weeber EJ, Rebeck W. Similarities and differences in structure, expression, and functions of VLDLR and ApoER2. Mol Neurodegener. 2011;6:30. doi: 10.1186/1750-1326-6-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda K, Ishida A, Takahashi K, Ueda T. Synaptic vesicles are capable of synthesizing the VGLUT substrate glutamate from alpha-ketoglutarate for vesicular loading. J Neurochem. 2012;121:184–196. doi: 10.1111/j.1471-4159.2012.07684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]