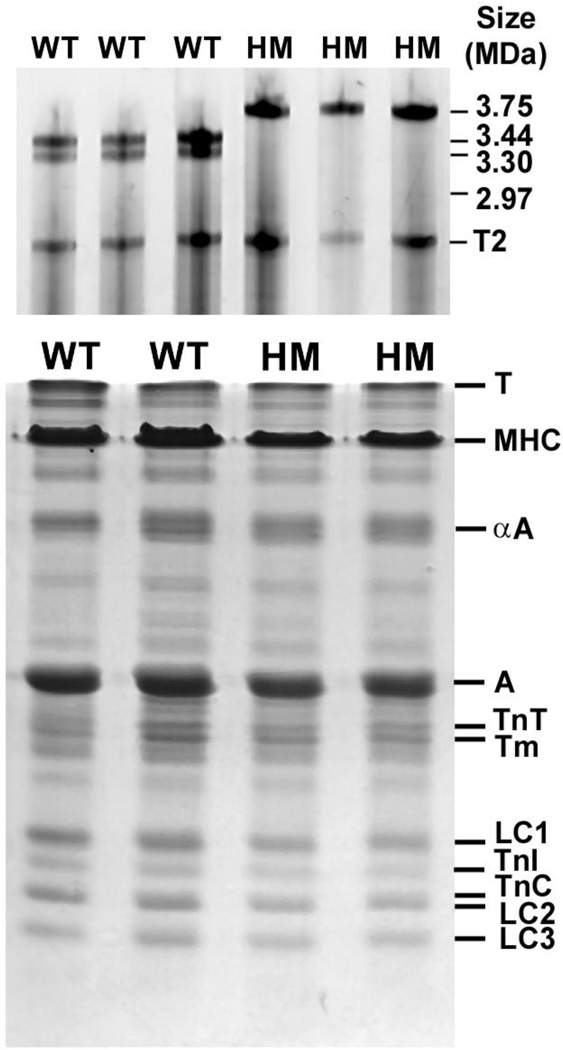
Fig. 1. Protein isoform composition SDS-PAGE analysis.
Tibialis anterior skeletal muscle (TA) was harvested from wild type (WT) and homozygous mutant (HM) rats. Top panel: shows agarose gel electrophoresis images used to analyze to titin isoform patterns. HM TA muscle expresses a single titin isoform that is significantly larger than the two major isoforms expressed in wild type TA muscle. T2 is a titin breakdown product which was similar in size between the WT and HM muscles. Bottom panel: myofilament contractile proteins were analyzed by standard 12% SDS-PAGE. There were no significant differences in contractile protein expression between WT and HM muscle. T, titin; MHC, myosin heavy chain; α-A, alpha actinin; A, actin; TnT, troponin T; Tm, tropomyosin; LC1, myosin light chain 1; TnI, troponin I; TnC, troponin C; LC2, myosin light chain 2; LC3, myosin light chain 3.