Fig. 1.
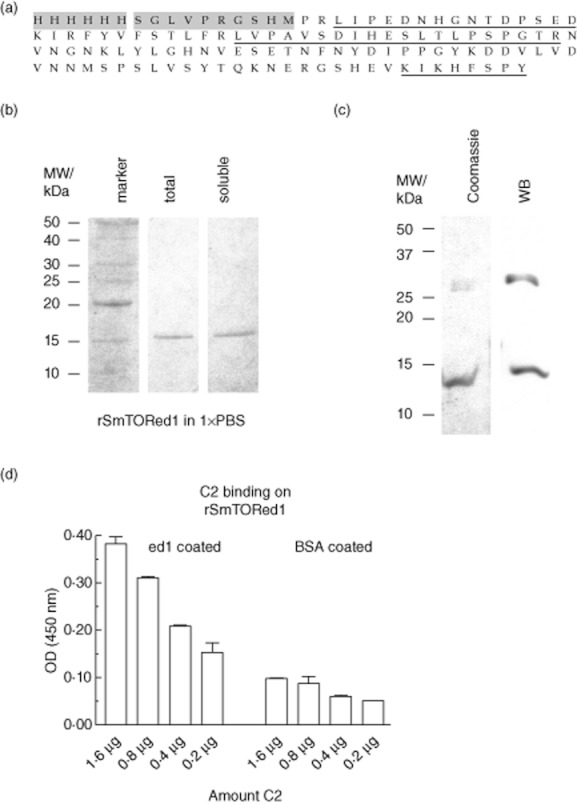
Production of histidine (His)-tagged Schistosoma mansoni tetraspanning orphan receptor extracellular domain 1 (SmTORed1) in Escherichia coli and molecular characterization of purified peptide by mass spectrometry, Western blotting and enzyme-linked immunosorbent assay (ELISA). (a) Recombinant first extracellular domain of rSmTORed1 peptide sequence with the 6 × His-tag and linker sequence (10 aa) originating from the pET15b vector shaded in grey. Fragments identified by mass spectrometry are underlined. (b) Sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) analysis of purified rSmTORed1 after Ni2+ chromatography (pooled eluates) and refolding into 1 × phosphate-buffered saline (PBS). Total amount of peptide in buffer was analysed before (total, lane 2) and after removal of residual precipitate by centrifugation (soluble, lane 3). Four μl of sample was loaded per lane. Lane 1 (marker): benchmark protein ladder. Protein bands are visualized by Coomassie stain. (c) Pooled fractions of fast protein liquid chromatography (FPLC)-purified peptide analysed before dialysis into 1 × PBS; 20 μl sample was loaded per lane. Peptides detected by Coomassie blue (left) and by Western blot using monoclonal anti-ed1 antibody AbDy04644·1 (right). (d) C2 binding on a rSmTORed1 coated ELISA plate, binding in 20 mM Tris buffer + 1 mM MgCl2 + 1 mM CaCl2. ELISA Results are expressed as means of individual measurements. Error bars indicate standard deviation (s.d.) of the means.
