FIG 1 .
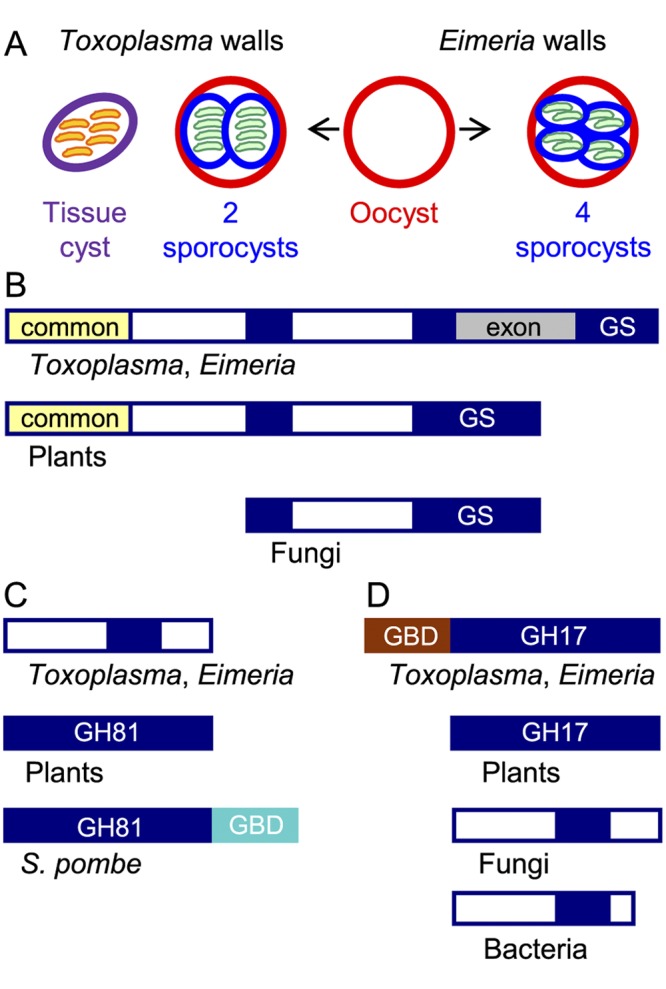
The glucan synthase and one glucan hydrolase of Toxoplasma gondii and Eimeria tenella resemble plant rather than fungal enzymes. (A) Toxoplasma has three walled forms: cat shed oocysts (red wall) in their feces, which sporulate in the environment to form two internal sporocysts (blue wall), each with four sporozoites (green). Tissue cysts (purple wall) in the muscle or brain of any warm-blooded animal contain numerous bradyzoites (orange). Chickens shed Eimeria oocysts in their feces that sporulate to form four sporocysts, each of which contains two sporozoites. (B) Toxoplasma and Eimeria glucan synthases have a conserved glucan synthase (GS) domain that is split into three parts (GS in blue), a domain common to plants, but not to fungi (yellow), and an exon (gray) present only in parasite glucan synthases. The white domains are unique to parasites, plants, or fungi. (C) One Toxoplasma and Eimeria glucan hydrolase contains a portion of the GH81 glycohydrolase domain present in plant and fungal enzymes but does not have a C-terminal glucan-binding domain (GBD in turquoise) present in the Schizosaccharomyces pombe (Eng1) glucan hydrolase. (D) The other Toxoplasma and Eimeria glucan hydrolase contains a GH17 glycohydrolase domain similar to that of plants and different from those of fungi and bacteria. The parasite glucan hydrolase also contains a glucan-binding domain (GBD in brown) between the signal peptide and the GH17 domain. The lectin activity of the TgGBD, which is not phylogenetically related to the SpGBD, is shown in Fig. 2. See Fig. S1 in the supplemental material for sequences of GBD and GH17 domains of the Toxoplasma glucan hydrolase.
