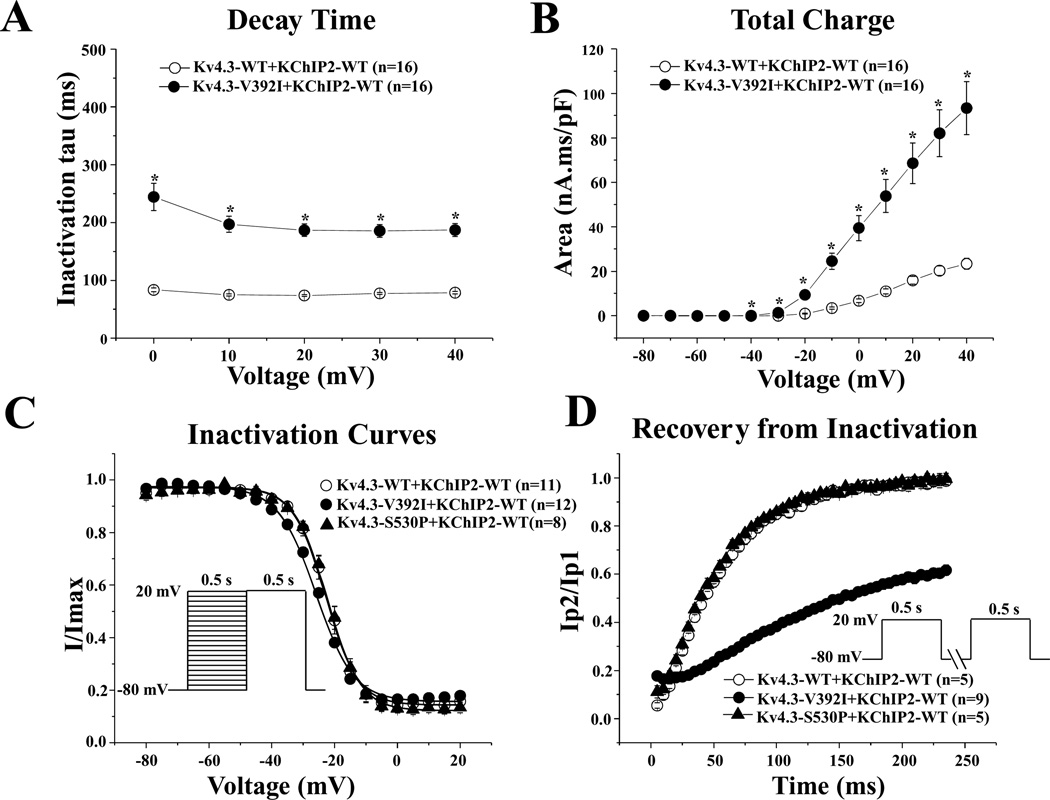
Figure 3.
Kinetic alteration for p.Val392Ile plus KChIP2. A: Inactivation time constants (τ) for wild-type (WT), or p.Val392Ile plus KChIP2 Kv4.3 currents as a function of voltage. Inactivation time constants for each voltage step were determined by fitting a monoexponential function to current decay. All values represent mean ± SEM. *P < 0.05 versus WT Kv4.3 plus KChIP2. B: Total charge of WT, or p.Val392Ile with KChIP2 as a function of voltage obtained by measuring the area under curve during the first 50 ms of each voltage step. *P < 0.05 versus WT Kv4.3 plus KChIP2. C: Steady-state inactivation curves of WT, p.Val392Ile, or p.Ser530Prp Kv4.3 with KChIP2 determined from a holding potential of −80 mV to prepulse of +20 mV in 5 mV increments with 0.5 s duration followed by a test pulse of +20 mV with 0.5 s duration and fitted with a Boltzmann function. D: Recovery from inactivation of WT, p.Val392Ile, or p.Ser530Pro Kv4.3 with KChIP2 determined from a holding potential of −80 mV to prepulse of +20 mV with 0.5 s duration, with increased recovery interval, followed by a test pulse of +20 mV with 0.5 s duration and fitted with a one-exponential function. All values shown represent mean ± SEM.