Abstract
Background:
Oral leukoplakia is the best-known precursor lesion. Although a morphologic feature of oral epithelial dysplasia is well described, less is known about the pathobiologic changes within the cells and over the cell surfaces for malignant transformation.
Aims:
The present study is aimed at comparing and correlating the mast cell density (MCD) and micro vascular density (MVD) in Normal Mucosa (NM) and different grades of dysplasia and to analyze their role in disease progression.
Materials and Methods:
MCD was assessed using anti mast cell tryptase and MVD was assessed immunohistochemically using anti-Factor VIII related von Willibrand factor.
Results:
The Results of the present study showed an exponential increase in microvessel density as mast cell density increased.
Conclusion:
The role of mast cells in angiogenesis as it progresses from normal mucosa to dysplasia is in concordance with the study. The number of mast cells and microvessel can be used as indictors of disease progression.
Keywords: Angiogenesis, mast cells, oral epithelial dysplasia
INTRODUCTION
The concept of a two-step process of cancer development in the oral mucosa, i.e. the initial presence of a precursor (pre-malignant, pre-cancerous) lesion subsequently developing into cancer, is well established. Oral leukoplakia is the best-known precursor lesion.[1]
Study of oral pre-malignant lesions can be a clue among the actions required for head and neck cancer prevention. Risk of malignant transformation of leukoplakia has been related to the kind of histological lesion.[2] The presence of epithelial dysplasia is generally accepted as one of the most important predictors of malignant development in pre-malignant lesions. Thus, the challenge within the field of oral pre-cancer is to predict which lesion will eventually develop in to carcinoma.[1]
Mast cells accumulate at sites of angiogenesis and its products facilitates angiogenesis. Thus, early accumulation of mast cells locally at a tissue site appears to potentiate the vessel growth. The newly recruited mast cells would consequently amplify and sustain the angiogenic process.[3]
Angiogenesis is a fundamental process in tumor growth and metastasis. It has been proposed that angiogenesis is needed for the growth of both primary and metastatic tumors and shows the demand for blood supply of lesion whose size increases beyond 1 or 2 mm. The induction of angiogenesis is mediated by positive and negative regulatory molecules released by both the tumor and host cells and depends on a net balance between positive and negative angiogenic factors.[4] This study aims to quantitate mast cell density (MCD) and microvessel density (MVD) in normal buccal mucosa (NM) and in oral pre-cancerous lesion – leukoplakia by immunohistochemistry using anti-mast cell tryptase and anti-factor VIII, respectively, and also to compare and correlate the MCD and MVD in NM and different grades of dysplasia and to analyze their role in disease progression.
MATERIALS AND METHODS
This study included 40 formalin fixed paraffin-embedded sections retrieved from the department of Oral and Maxillo-facial Pathology, Meenakshi Ammal Dental College and Hospital, Chennai. Thirty cases of histologically diagnosed oral epithelial dysplasia (OED) were selected and the clinical data of the same such as age, gender, personal habits were taken from the records. It was then confirmed for the histological diagnosis which was earlier given. Ten cases were of clinically normal oral buccal mucosa of individuals without any habit formed the control group. Clinical data and informed consent were taken from these patients. Five of the cases of controls were taken from left buccal mucosa and the other five from right buccal mucosa.
Controls
Control section for staining included neurofibroma for mast cells and pyogenic granuloma for angiogenesis formed the positive control and were treated in the same manner as the test groups and one normal buccal mucosa and one leukoplakia lesion forming the negative control were treated in the same manner as the test groups except that the primary antibody was omitted and substituted with phosphate-buffered saline and a non-immune antibody (normal rabbit serum) at the same concentration.
Staining
H and E staining
Formalin fixed paraffin-embedded tissues were sectioned at 4 μm thickness and taken for routine H and E staining. These sections were used for grading of dysplasia in oral leukoplakia cases using the binary system given by Kujan et al.
Immunohistochemical staining
The serial sections of the same were stained by immunohistochemical reagents, anti-MC tryptase for mast cells, and anti-factor VIII related von Willebrand Factor for endothelial cells (Biogenex, San Ramon, CA).
Methodology
Four micrometer thin sections were taken on to slides which were kept in 4% chromic acid and coated with amino-propyl ethoxy silane (APES) and were dried in an microwave oven at 60°C overnight. Sections were deparaffinized and were subjected to antigen retrieval in 10M citrate acid buffer solution and boiled thrice in microwave oven for 5 min. The solution is allowed to cool to room temperature and washed in Tris buffer saline (TBS).
Sections were further treated with methanemic hydrogen peroxide for 10 min at room temperature to block the endogenous peroxidase activity. Power block was performed for 15 min to block background staining. Primary monoclonal antibody anti-mast cell tryptase (Biogenex, San Ramon, CA) was incubated for 1 h for slides to be stained for mast cells, and anti-factor VIII (Biogenex, San Ramon, CA) related von willebrand factor for 2 h followed by secondary antibody – super enhancer and PolyHRP (Biogenex, San Ramon, CA) for 30 min. Excess is wiped off and after wash with PBS for two changes; sections were incubated with DAB substrate chromogen (Biogenex, San Ramon, CA) for 15-20 min. They were counterstained with 1 dip Harris hematoxylin followed by bluing in running tap water. Slides were air dried and mounted with DPX.
Evaluation
Mast cells and microvessels were counted in the systematic field at ×400 using oculometer grid which had 100 subdivisions. Four to five fields at ×400 (0.0625 mm2) were counted and the mean average of the fields were considered for the counting. The criteria for counting the mast cells were that any cluster of mast cell granule positive for anti-MC tryptase and clearly separate from an adjacent cluster was considered to be a single mast cell and appearing brown in color; cellular boundary was not necessary for a structure to be defined as mast cell [Figures 1–3]. Similarly for the blood vessel, any endothelial-lined vessel lumen or endothelial cell cluster positive for factor VIII-related antigen, i.e. stained brown and clearly separate from an adjacent cluster was considered to be a single, countable microvessel [Figures 4–6]. Vessel lumen was not necessary for a structure to be defined as a microvessel and red blood cells were not used to define a vessel lumen. The mean number of mast cells and microvessels were calculated using the formula.
Figure 1.
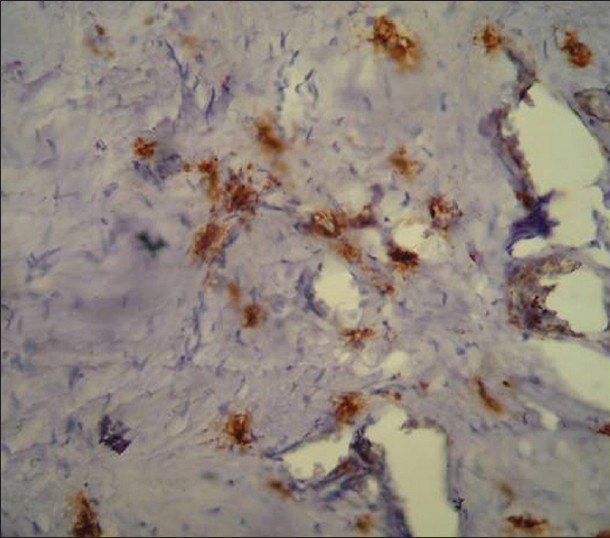
Immunohistochemical demonstration of mast cells in normal mucosa using mouse monoclonal anti-mast cell tryptase antibody (×400)
Figure 3.
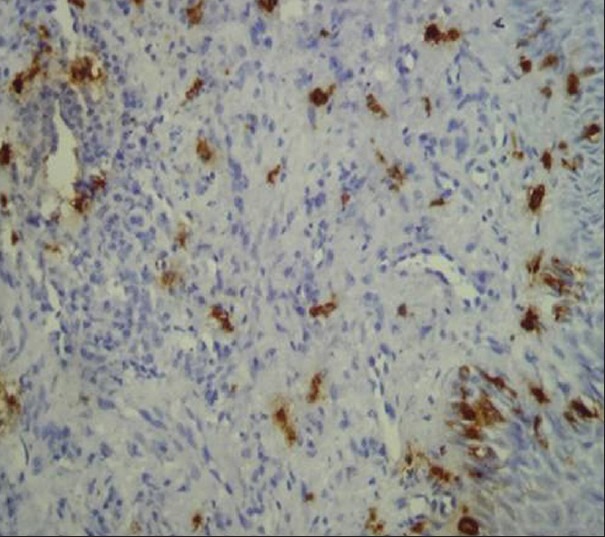
Immunohistochemical demonstration of mast cells in high grade dysplasia using mouse monoclonal anti-mast cell tryptase antibody (×400)
Figure 4.
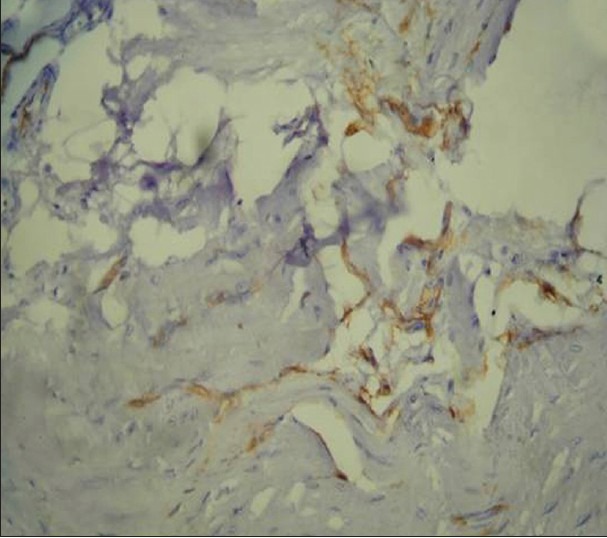
Immunohistochemical demonstration of microvessels in normal mucosa using mouse monoclonal anti-factor VIII related Von willebrand factor antibody (×400)
Figure 6.
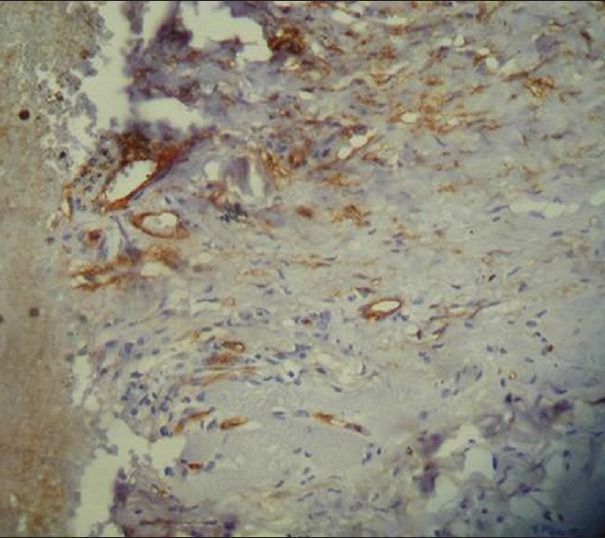
Immunohistochemical demonstration of microvessels in high grade dysplasia using mouse monoclonal anti-factor VIII related Von willebrand factor antibody (×400)
Figure 2.
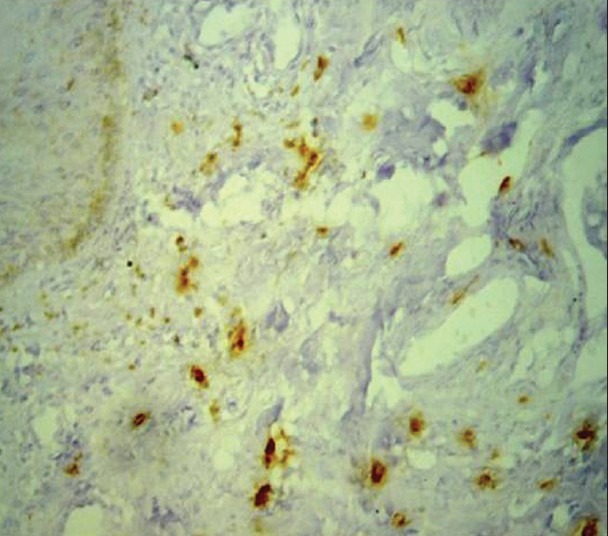
Immunohistochemical demonstration of mast cells in low grade dysplasia using mouse monoclonal anti-mast cell tryptase antibody (×400)
Figure 5.
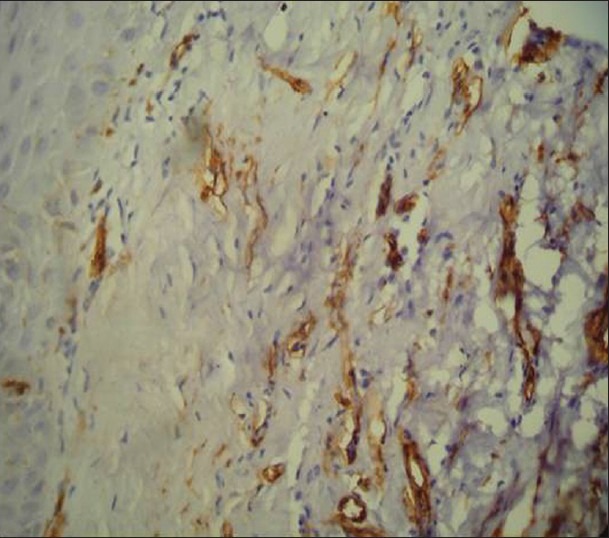
Immunohistochemical demonstration of microvessels in low grade dysplasia using mouse monoclonal anti-factor VIII related Von willebrand factor antibody (×400)
The number of mast cells per sq. mm was calculated using the formula:
![]()
Similarly, the number of microvessels per sq. mm was calculated using the formula:

Further, the mean values are subjected to statistical analysis using independent ‘t’- test, ANOVA, Tukey HSD and correlation of MCD with MVD is done using Pearson's correlation test.
RESULTS
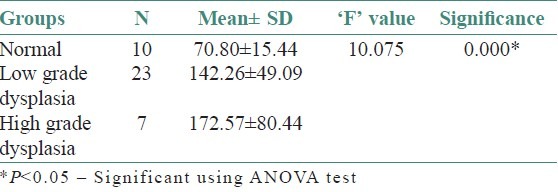
On evaluating the control sections, the mean mast cell and MVD using independent ‘t’- test were 114.40/mm2 and 70.80/mm2, respectively. All leukoplakia cases were divided into two groups as low-grade dysplasia (LGD) and high-grade dysplasia (HGD) based on the binary classification by Kujan (2006) et al. The mean mast cell and MVD for LGD were 168.86/mm2 and 142.26/mm2 respectively. Similarly, for HGD, it was 193.71/mm2 and 172.57/mm2 respectively. Comparison showed a significant increase in the MCD and MVD among LGD and HGD when compared to normal cases (P < 0.05) using ANOVA and Tukey HSD [Tables 1–4]. But the increase in MCD and MVD between LGD and HGD is not statistically significant (P > 0.05). Correlation analysis revealed a positive correlation in LGD between MCD and MVD and was highly significant (P < 0.01) [Figure 7]. As MCD increases, there is an exponential increase in MVD. Similarly, correlation analysis revealed a positive correlation between MCD and MVD but was not statistically significant (P > 0.01) in NM and HGD.
Table 1.
One way comparison of mean mast cell density
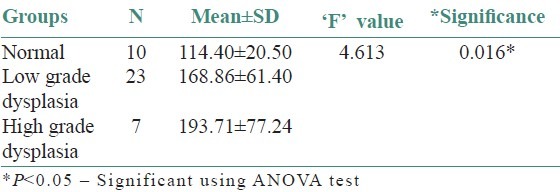
Table 4.
Inter group comparison of microvessel density
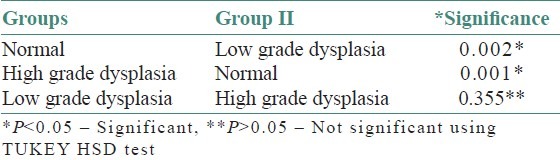
Figure 7.
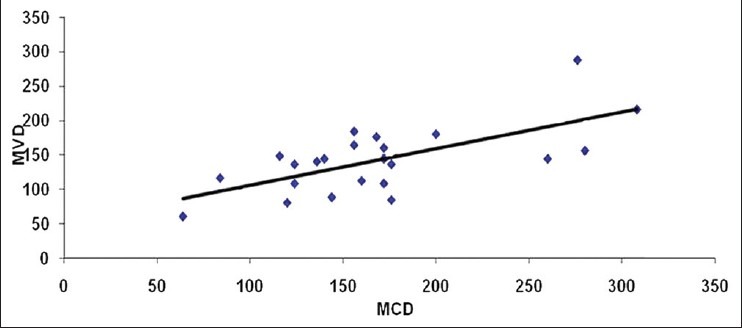
Scattered diagram showing correlation of MCD and MVD in low grade dysplasia
Table 2.
Inter group comparison of mast cell density
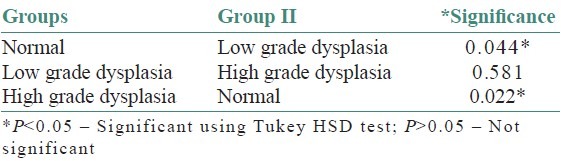
Table 3.
One way comparison of microvessel density
Correlation is highly significant using Pearson's correlation and shows an exponential increase between MCD and MVD.
DISCUSSION
It has been reviewed by many workers that oral dysplastic lesions can progress to carcinoma. The work of Speight and Morgan has shown that approximately 10-20% of dysplastic lesions become malignant within 10 years, 20-30% increase in severity, 0-13% regress, and the remainder shows no change. The overall chance for change from dysplastic to malignancy is 11%. However, in severe dysplasia, it rises to 43%.[5]
OED's may be morphological phenotypes of different steps in progression from normal to malignant tissue. OED was considered the progenitor for malignant changes. OED was classified according to WHO (2003) as mild, moderate, severe, or carcinoma in situ according to the presence and severity of cellular atypia and to architectural changes based on the thickness of the layers. Kujan et al. (2006)[6] proposed and evaluated a new scheme based on the same morphological criteria used by WHO classification (2005 architectural and cytology changes) that grades the lesions into either low risk or high risk based on the scoring features.[6] However, in using the new two-scale grading system, it distinguishes between two types of moderate dysplasia based on the clinical outcomes. Thus, the use of binary system prevents the risk of underestimation and also overgrading.[5,7] Therefore, we adapted the binary system of grading of Kujan et al. (2006)[6] for our study.
In our study, out of 30 cases, 23 were belonging to the low risk and 7 were of high risk.
In relation to the staining techniques for mast cells, both toluidine blue and immunohistochemistry identification techniques reliably identify MC granules; a recent study by Olieivario et al. (2007)[8] showed that the immunohistochemical method is more specific than metachromatic staining by toluidine blue.
In this study, average number of mast cells/mm2 was significantly more for dysplastic epithelium as compared to NM. Similarly, MCD showed highly significant increase from NM to LGD to HGD. This result was consistent with the finding of Biviji et al. (1973)[9] and Ankle et al. (2007)[10] At present, it remains unclear whether mast cells move into these sites as a result of migration of fully differentiated mast cells from nearby sites or differentiate from precursors already located at the site.
Poole et al. (1983)[11] investigated the migratory response of rat peritoneal mast cells to factors released from a variety of normal and tumor-derived cells. Using a chemotaxis assay system under agarose, they demonstrated that mast cells move toward chemoattractants produced by each of the tumor cell types, but normal cells failed to produce such factors.[12]
In many normal tissues, mast cells are identified as being “intact,” compatible with their surveillance role. Local tissue hemostasis including cell division might well depend on the controlled production and release of specific MC-derived factors, host of endogenous non-IgE-mediated secretagogues, such as venom, bacterial stimuli, drugs, neuroenteric peptides which form the basis for non-immune stimuli and immune stimuli which include IgE mediated, C3a, C4a, C5a, IL-1, and TNF secretagogues are recognized.[12,13]
Carcinogens presence may induce cytotoxicity, mutagenicity, and genotoxicity toward different kinds of cells including oral epithelial cells and induce oral keratinocytes to secrete TNF-α, IL-6, PGE2 which might provoke oral mucosal inflammation. N’nitrosonornicotine in chewing tobacco; polycyclic hydrocarbon, precarcinogens in the tobacco smoke,[14] and 4-nitroqunoline-N-oxide, a carcinogen which induced tumors in experimental rats showed an evident infiltrate of macrophages, T cells.[15]
It is possible that these may be the factors which we consider to have a stimulatory effect on mast cells as a non-immune-mediated mechanism.
It is found that an increase in relative vascular volume in the stroma of pre-malignant lesion and malignant lesions of the oral cheek lesions is accompanied by both angiogenesis and vasodilatation of the blood vessels which may reflect the increasing nutrient requirements of actively growing and dividing cells.[16]
Search for endothelial markers widened to include the application of αvβ3 which is considered more specific. However, the study conducted by Pazouki et al. (1997)[5] used pan endothelial markers (vWF, CD31) as well as αvβ3. They found similar Results with vWF and CD31. However, they considered that the expression of αvβ3 has limited diagnostic value and does not appear to reflect the presence of angiogenic vessels.[5]
In this study, MVD was assessed in different histological grades of oral dysplasia. MVD showed highly significant increase from NM to LGD to HGD. However, the increase in MVD between LGD and HGD was not statistically significant. Our Results are consistent with the study done by Jin et al. (1995),[16] Carlile et al. (2001),[17] Cedeno et al. (2005),[18] Abbas et al. (2007),[19] Raica et al. (2009),[20] and Mohtasham et al. (2010).[21] Similarly, our Results were in contrast to the study done by Tae et al. where in, MVD in pre-malignant lesion showed no correlation with histological grade.[22]
Our study demonstrated positive correlation between MCD and MVD; as MCD increases, there is an exponential increase in MVD.
These findings are in line with previous studies of Iamaroon et al. (2003)[23] in OSCC. This finding suggests that mast cells may upregulate tumor angiogenesis via secretion of angiogenic factors, including histamine, heparin, tryptase, vascular endothelial growth factor, and basic fibroblast growth factor.[13,23]
A key mediator that influences mast cell migration is mast cell growth factor (MGF). This molecule can be synthesized by endothelial cells (Weiss et al., 1995)[24] and epidermal keratinocytes (Klighman et al., 1996)[25] and is thought to direct the homing of mast cell precursors to epithelial tissues.
Nitric oxide (NO) is a gaseous free radical, generated by the action of nitrous oxide synthase (NOS), and causes net vasodilatation. Mucosal inflammation is associated with induction of inducible NOS (iNOS) in epithelial cells and to a much lesser extent in inflammatory cells of the lamina propria. It has been suggested that the interaction of tumor cells and macrophages might cause activation of the enzyme in the tumor probably caused by direct contact or contingent paracrine activating factor.[26]
But, in our observation, the increase from LGD to HGD of both the MCD and MVD was not statistically significant. This may be due to the disparity between the number of LGD and HGD in which the sample number is very small.
CONCLUSION
To conclude, the Results of this study showed an exponential increase in MVD as MCD increases, thus revealing the role of mast cells in angiogenesis as it progresses from NM to dysplasia. The number of mast cells and microvessel can be used as indicators of disease progression. Further studies are essential with an increased sample size of oral leukoplakia which includes equal numbers of both LGD and HGD for the practical application.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Reibel J. Prognosis of oral pre-malignant lesions: Significance of clinical, histopathological, and molecular biological characteristics. Crit Rev Oral Biol Med. 2003;14:47–62. doi: 10.1177/154411130301400105. [DOI] [PubMed] [Google Scholar]
- 2.Santos-García A, Abad-Hernández MM, Fonseca-Sánchez E, Cruz-Hernández JJ, Bullón-Sopelana A. Proteic expression of p53 and cellular proliferation in oral leukoplakias. (1-5).Med Oral Patol Oral Cir Bucal. 2005;10:5–8. [PubMed] [Google Scholar]
- 3.Cairns JA, Walls AF. Mast cell tryptase is a mitogen for epithelial cells.Stimulation of IL-8 production and intercellular adhesion molecule-1 expression. J Immunol. 1996;156:275–83. [PubMed] [Google Scholar]
- 4.Kumar V, Abbas AK, Fausto N. Tissue renewal and repair: Regeneration, Healing and fibrosis. In: Robbins, Cotran, editors. Pathologic Basis of Disease. 7th ed. Philadelphia: W.B. Saunders Company; 2006. p. 106. [Google Scholar]
- 5.Pazouki S, Chisholm DM, Adi MM, Carmichael G, Farquharson M, Ogden GR, et al. The association between tumour progression and vascularity in the oral mucosa. J Pathol. 1997;183:39–43. doi: 10.1002/(SICI)1096-9896(199709)183:1<39::AID-PATH1088>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 6.Kujan O, Oliver RJ, Khattab A, Roberts SA, Thakker N, Sloan P. Evaluation of a new binary system of grading oral epithelial dysplasia for prediction of malignant transformation. Oral Oncol. 2006;42:987–93. doi: 10.1016/j.oraloncology.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 7.van der Waal I, Schepman KP, van der Meij EH, Smeele LE. Oral leukoplakia: A clinicopathological review. Oral Oncol. 1997;33:291–301. doi: 10.1016/s1368-8375(97)00002-x. [DOI] [PubMed] [Google Scholar]
- 8.Oliveira-Neto HH, Leite AF, Costa NL, Alencar RC, Lara VS, Silva TA, et al. Decrease in mast cells in oral squamous cell carcinoma: Possible failure in the migration of these cells. Oral Oncol. 2007;43:484–90. doi: 10.1016/j.oraloncology.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 9.Biviji AT. Mast cells in normal and leukoplakic buccal mucosa. J Indian Dent Assoc. 1973;45:189–91. [PubMed] [Google Scholar]
- 10.Ankle MR, Kale AD, Nayak R. Mast cells are increased in leukoplakia, oral submucous fibrosis, oral lichen planus and oral squamous cell carcinoma. J Oral Maxillofac Pathol. 2007;11:18–22. [Google Scholar]
- 11.Poole TJ, Zetter BR. Stimulation of rat peritoneal mast cell migration by tumor-derived peptides. Cancer Res. 1983;43:5857–61. [PubMed] [Google Scholar]
- 12.Norrby K, Woolley D. Role of mast cells in mitogenesis and angiogenesis in normal tissue and tumor tissue. Adv Biosci. 1993;89:71–116. [Google Scholar]
- 13.Rothe M J, Nowak M, Kerdel FA. The mast cell in health and disease. J Am Acad Dermatol. 1990;23:615–24. doi: 10.1016/0190-9622(90)70264-i. [DOI] [PubMed] [Google Scholar]
- 14.Pillai R, Balaram P, Reddiar KS. Pathogenesis of oral submucous fibrosis. Cancer. 1992;69:2011–20. doi: 10.1002/1097-0142(19920415)69:8<2011::aid-cncr2820690802>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 15.Chang MC, Chiang CP, Lin CL, Lee JJ, Hahn LJ, Jeng JH. Cell-mediated immunity and head and neck cancer: with special emphasis on betel quid chewing habit. Oral Oncol. 2005;41:757–75. doi: 10.1016/j.oraloncology.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 16.Jin Y, Tipoe GL, White FH, Yang L. A quantitative investigation of immunocytochemically stained blood vessels in normal, benign, premalignant and malignant human oral cheek epithelium. Virchows Arch. 1995;427:145–51. doi: 10.1007/BF00196519. [DOI] [PubMed] [Google Scholar]
- 17.Carlile J, Harada K, Baililie R, Mackluskey M, Chisholm DM, Ogden GR, et al. Vascular endothelial growth factor (VEGF) expression in oral tissues: Possible relevance to angiogenesis, tumor progression and field cancerisation. J Oral Pathol Med. 2001;30:449–57. doi: 10.1034/j.1600-0714.2001.030008449.x. [DOI] [PubMed] [Google Scholar]
- 18.Cedeno F, Tonino P. Angiogenesis in premalignant and malignant lesions of the oral mucosa. Act Odontol Venez. 2005;43:108–12. [Google Scholar]
- 19.Abbas NF, El-Sharakawy SL, Abbas EA, El-sahaer MA. Immunohistochemical study of p53 and angiogenesis in benign and preneoplastic oral lesions and oral squamous cell carcinoma. Oral surg oral med Oral Pathol Oral Radiol Endod. 2007;103:385–90. doi: 10.1016/j.tripleo.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 20.Raica M, Cimpean AM, Ribatti D. Angiogenesis in pre-malignant conditions. Eur J Cancer. 2009;45:1924–34. doi: 10.1016/j.ejca.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 21.Mohtasham N, Babakoohi S, Salehinejad J, Montaser-Kouhsari L, Shakeri MT, Shojaee S, et al. Mast cell density and angiogenesis in oral dysplastic epithelium and low- and high-grade oral squamous cell carcinoma. Acta Odontol Scand. 2010;68:300–4. doi: 10.3109/00016357.2010.494622. [DOI] [PubMed] [Google Scholar]
- 22.Tae K, El-Naggar AK, Yoo E, Feng L, Lee JJ, Hong WK, et al. Expression of vascular endothelial growth factor and microvessel density in head and neck tumorigenesis. Clin Cancer Res. 2000;6:2821–8. [PubMed] [Google Scholar]
- 23.Iamaroon A, Pongsiriwet S, Jittidecharaks S, Pattanaporn K, Prapayasatok S, Wanachantararak S. Increase of mast cells and tumor angiogenesis in oral squamous cell carcinoma. J Oral Pathol Med. 2003;32:195–9. doi: 10.1034/j.1600-0714.2003.00128.x. [DOI] [PubMed] [Google Scholar]
- 24.Weiss RR, Whitaker-Menezes D, Longley J, Bender J, Murphy GF. Human dermal endothelial cells express membrane-associated mast cell growth factor. J Invest Dermatol. 1995;104:101–6. doi: 10.1111/1523-1747.ep12613587. [DOI] [PubMed] [Google Scholar]
- 25.Kligman LH, Murphy GF. Ultraviolet B radiation increases hairless mouse mast cells in a dose-dependent manner and alters distribution of UV-induced mast cell growth factor. Photochem Photobiol. 1996;63:123–7. doi: 10.1111/j.1751-1097.1996.tb03002.x. [DOI] [PubMed] [Google Scholar]
- 26.Rajendran R, Varkey S. Inducible nitric oxide synthase expression is upregulated in oral submucous fibrosis. Indian J Dent Res. 2007;18:94–100. doi: 10.4103/0970-9290.33783. [DOI] [PubMed] [Google Scholar]


