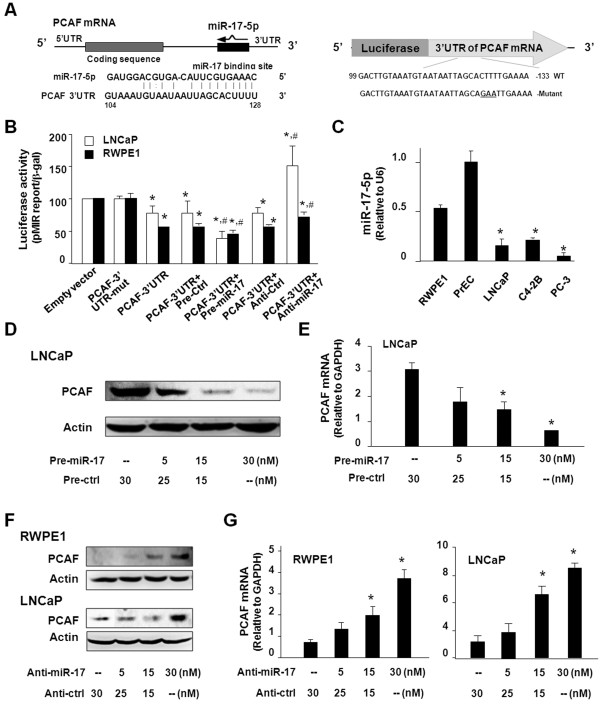
Figure 3.
miR-17-5p targets PCAF 3’UTR, resulting in translational suppression and RNA degradation. A, the schematic of human PCAF mRNA showed a potential binding site in its 3’UTR for miR-17-5p. The complementary miR-17-5p-binding site in the PCAF 3’UTR was inserted to the downstream of a luciferase reporter on the pMIR-REPORT plasmid. A control plasmid with the mutant 3’UTR sequence was also generated. WT = wild-type. B, binding of miR-17-5p to the potential binding site in the PCAF 3’UTR results in translational suppression, as assessed by luciferase reporter assay. LNCaP and RWPE1 cells were transfected with the plasmids and treated with the anti-miR or precursor to miR-17-5p, or non-specific oligo control, for 24 h, followed by luciferase analysis. Mut = mutant; *, p < 0.05 compared to the controls; #, p < 0.05 compared to PCAF 3’UTR transfected alone. C, expression of miR-17-5p in cells, as assessed by qRT-CR. Mature miR-17-5p level was obtained by normalizing to the endogenous reference RNU6B. D and E, miR-17-5p precursor decreases PCAF expression in LNCaP cells. Cells were treated with various doses of miR-17-5p precursor or nonspecific precursor control, followed by Western blot for PCAF protein (after incubation for 48 h) or PCR for PCAF mRNA (after incubation for 24 h). F and G, anti-miR-17-5p increases PCAF expression in RWPE1 and LNCaP cells. Cells were treated with various doses of anti-miR-17-5p or non-specific anti-miR control followed by Western blot for PCAF protein (48 h) or PCR for PCAF mRNA (24 h). Data in B, C, E, and G are averages of three independent experiments. *, p < 0.05 compared to RWPE1 and PrEC cells (in C) or the controls in E and G.