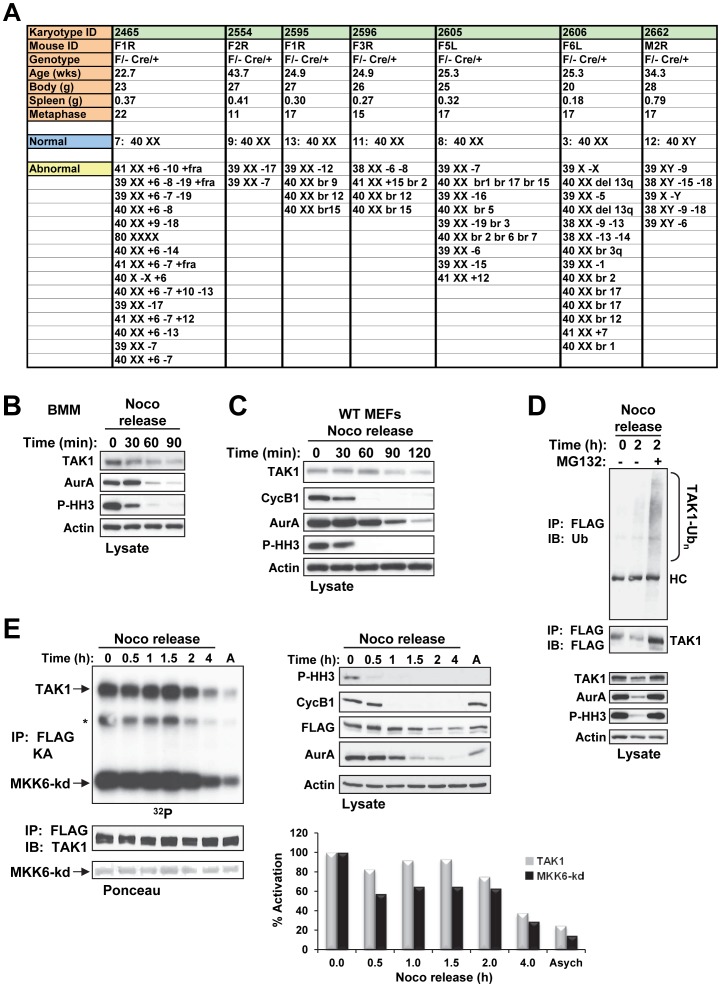
Figure 9. Diseased TAK1ΔM Mice Exhibit Genomic Instability and TAK1 is regulated during mitosis.
(A) TAK1ΔM mice were euthanized because of morbidity, and body and spleen weights were measured. Bone marrow was harvested, and short-term cultures were collected and processed for G-banding using standard methods. Analysis of 11–22 metaphase cells per mouse was performed, and the results listed indicated chromosomal deletions, additions, or breaks. (B–C) Primary mouse BMMs (B) or TAK1-WT MEFs (C) were treated with nocodazole (Noco, 100 ng/ml) for 14 h. Mitotic cells were collected by the mitotic shake-off method and released into fresh medium for the indicated times. Cell lysates were subjected to SDS-PAGE and immunoblotted with the indicated antibodies. (D) TAK1-KO MEFs stably expressing FLAG-TAK1 were treated with nocodazole (100 ng/ml) for 14 h. Mitotic cells were collected by the mitotic shake-off method and released into fresh medium for the indicated times in the absence or presence of MG132 (25 µM; added 30 min after nocodazole release). Cell lysates were subjected to SDS-PAGE and immunoblotted with the indicated antibodies (bottom panels). Protein lysates were immunoprecipitated with anti-FLAG and immunoblotted with anti-ubiquitin (Ub) as indicated (top panel). The membrane was stripped and reprobed with anti-FLAG (middle panel). (E) TAK1-KO MEFs stably expressing FLAG-TAK1 were left untreated (A; asynchronized) or treated with nocodazole (100 ng/ml) for 14 h. Mitotic cells were collected by the mitotic shake-off method and released into fresh medium for the indicated times. Cells lysates were subjected to SDS-PAGE and immunoblotted with the indicated antibodies (right panels). Protein lysates were immunoprecipitated with anti-FLAG and an in vitro kinase assay with kinase-dead MKK6 (MKK6-kd) as the substrate was performed. The amount of total protein was adjusted so that an equivalent amount of TAK1 was immunoprecipitated from each time. The samples were subjected to SDS-PAGE, transferred to a membrane, and exposed to X-ray film (upper left panel). The membrane was stained with Ponceau S (bottom left panel) and probed with anti-TAK1 (middle left panel). Incorporation of 32P into the substrate and TAK1 was quantitated using a PhosphoImager and presented as percent activation (graph). Asych, asynchronized.