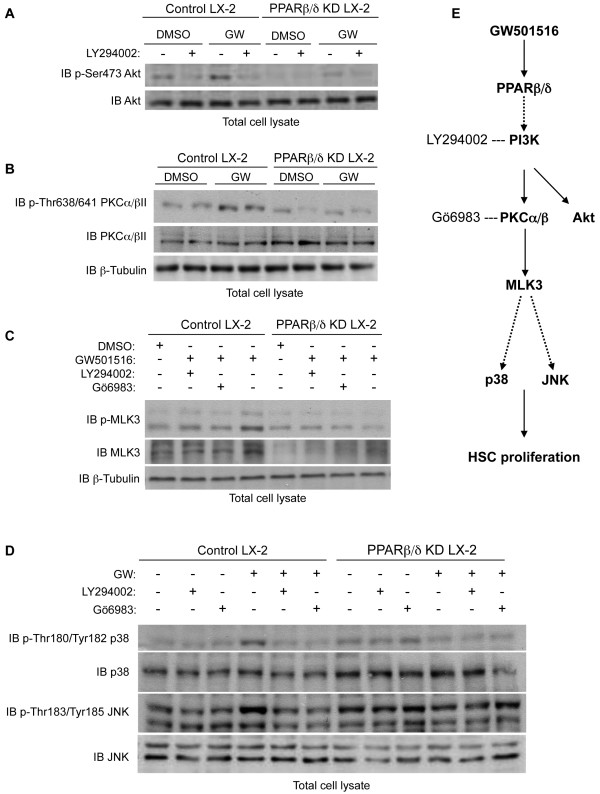
Figure 7.
PPARβ/δinduced a signaling pathway involving PI3K/PKCα/βII/MLK3/p38andJNK MAPKs. Control and PPARβ/δ KD LX-2 cells were serum-starved for 24 h, and then pre-treated with the indicated inhibitor for 30 min before incubation with 100 nM GW501516 or 0.01% DMSO. After total cell lysis, proteins were resolved by immunoblot (IB).β-Tubulin served as the loading control. A) IB shows phosphorylation of Akt on Ser473 in the presence or absence of PI3K inhibitor LY294002 (20 μM). B) IB shows PPARβ/δ-dependent PKCα/βII protein expression and phosphorylation. C) IB shows MLK3 protein expression and phosphorylation with or without PI3K inhibitor LY294002 (20 μM) or PKC inhibitor Gö6983 (7 μM). D) IB shows p38 and JNK protein expression and phosphorylation in the presence or absence of LY294002 (20 μM) or Gö6983 (7 μM). IBs are representative of three independent experiments. E) Schematic model for the regulation of human hepatic LX-2 stellate cell proliferation by GW501516-activated PPARβ/δ. Ligand activation of PPARβ/δ enhances PI3K activity, resulting in activation of PKCα/βII and downstream MLK3. MLK3 signaling eventually results in increased phosphorylation of p38 and JNK MAPKs, which are known to enhance HSC proliferation.