Figure 7. Comparison of the binding of the different porphyrins to 13G10 and 14H7 and models of imidazole binding to the 13G10/porphyrin complexes.
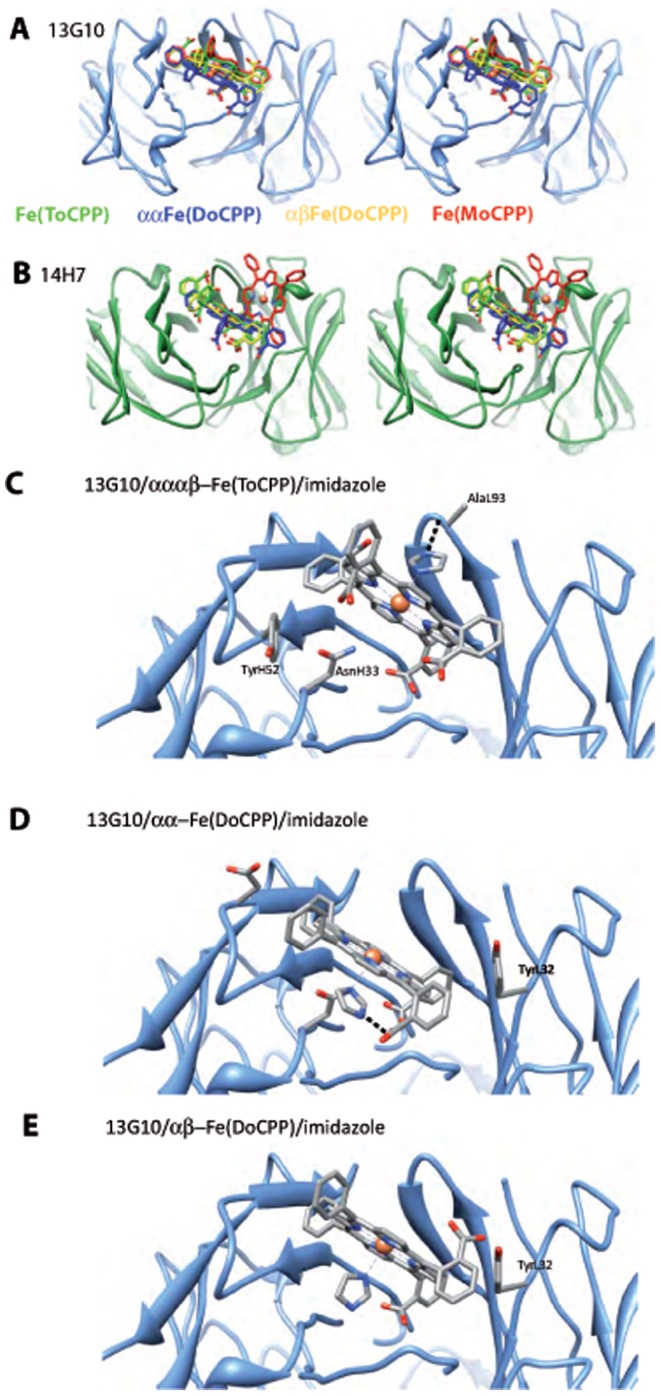
Stereoviews of the superposition of the different porphyrins in the combining sites of 13G10 (A) and 14H7 (B). Fe(ToCPP), αα-Fe(DoCPP), αβ-Fe(DoCPP) and Fe(MoCPP) are colored green, blue, yellow and red, respectively. For 13G10, the porphyrins of Fe(MoCPP), αα-Fe(DoCPP) and αβ-Fe(DoCPP) superimpose onto that of Fe(ToCPP)(without taking into account the carboxylate substituents) with rmsds of 0.5, 3.0 and 1.7 Å2, respectively. For 14H7, the porphyrins of Fe(MoCPP), αα-Fe(DoCPP) and αβ-Fe(DoCPP) superimpose onto that of Fe(ToCPP) with rmsds of 9.1, 3.1 and 0.9 Å2, respectively. C Model of imidazole bound to 13G10/Fe(ToCPP). The binding of the imidazole molecule is favored by an H-bonding interaction with the main chain of AlaL93. D Model of imidazole bound to 13G10/αα-Fe(DoCPP). Despite some clashes, imidazole binding is stabilized by H-bonds with AsnH33 and one of the buried carboxylates. Stabilization is also achieved by hydrophobic contacts as imidazole binds in the cavity left between the cofactor and the protein. E Model of imidazole bound to 13G10/αβ-Fe(DoCPP). Imidazole is located on the buried side of the porphyrin but does not make any interaction with the protein, the β-carboxylate being oriented toward the solvent.
