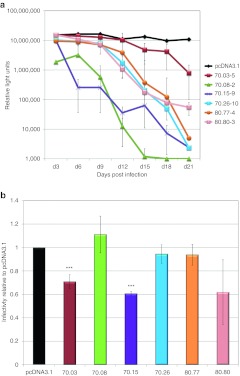
Figure 1.
Aptamer-mediated inhibition of human immunodeficiency virus (HIV) replication in multiple-cycle and single-cycle assays when aptamers are expressed from the mcHHRz cassette. (a) Multiple-cycle assays. HIV-1NL4-3 serial passage using an initial multiplicity of infection (MOI) of 0.6 in clonal, stable TZM-bl cell lines made by transfection and subsequent G418 selection with the mcHHRz aptamer-expression constructs expressing aptamers 70.03, 70.08, 70.15, 70.26, 80.77, and 80.80. The same number of cells for each line were plated in quadruplicate for each passage, using three wells for infection and one well as a noninfected control for background luciferase activity. One-half of the virus-containing supernatant was transferred in series to fresh cells every 3 days, and infectivity was determined using BetaGlo reagent to quantify luciferase activity. Background luciferase activity in the absence of virus was subtracted and values are shown ±SD for three replicates. (b) Single-cycle assays. mcHHRz aptamer-expression constructs were transfected into 293FT cells in triplicate, followed four hours later by media change and cotransfection of pNL4-3-CMV-EGFP and pMD-G. Virus-containing supernatants were harvested 48 hours after the final media change and 100 µl was used to infect fresh 293FT cells at a density of 50%. Infectivity was determined using flow cytometry and is shown as % EGFP-positive cells normalized to the negative control, pcDNA3.1. One-way ANOVA analysis and Student's t-test were used to determine significance compared to pcDNA3.1 controls (P < 0.001). Values are shown as the mean ±SD for three experiments. CMV, cytomegalovirus; EGFP, enhanced green fluorescent protein.