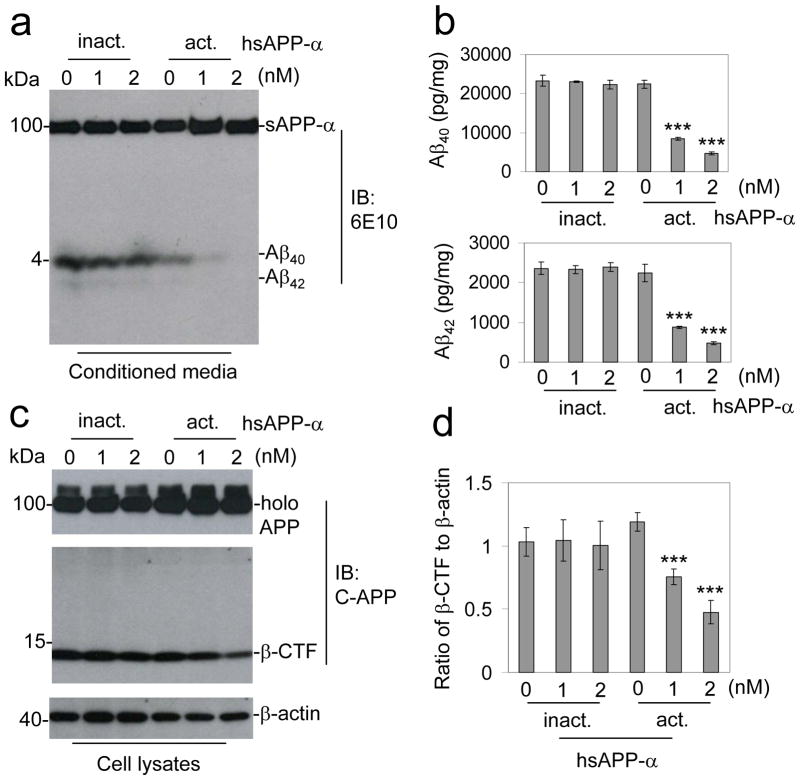
Figure 1.
sAPP-α inhibitsβ-secretase cleavage of APP in vitro. CHO cells expressing APPswe and wild-type human PS1 (CHO/APPswe/PS1wt) cells were treated with active (act.) or inactive (inact.) hsAPP-α recombinant protein at 0, 1, and 2 nM as indicated for 4 h. Secreted Aβ40, 42 peptides in the cell culture medium were analyzed by (a) immunoblot (IB) and (b) Aβ ELISA analyses. The Aβ ELISA results are represented as the mean ± SD of picograms of Aβ40 or Aβ42 per milligram of total intracellular protein after hsAPP-α protein treatment. In addition, these results are representative of four independent experiments with n = 3 for each condition. (c) Cell lysates were prepared and APP CTFs were analyzed by IB using a rabbit polyclonal antibody against C-terminal APP (pAb751/770, C-APP). This β-CTF band was further confirmed by the additional IB using 6E10 antibody against Aβ1-17 peptide for sAPP-α. (d) Relative ratio (mean ± SD) of β-CTF to β-actin was calculated by densitometry analysis. The results are representative of three independent experiments with n = 3 for each condition. One-way ANOVA followed by post hoc comparison revealed significant differences between 1 or 2 and 0 nM hsAPP-α protein treatment in both of Aβ40, 42 reduction and relative ratio of β-CTF to β-actin. (***P < 0.001, Full-length APP: holo APP)