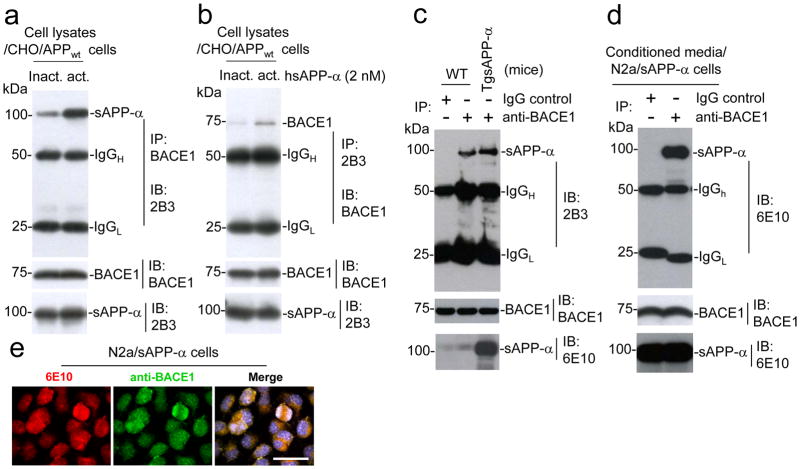
Figure 4.
sAPP-α interacts with BACE1 in cultured cells. CHO/APPwt cells were treated with active or inactive hsAPP-α protein at 2 nM for 8 h. The cell lysates were immuoprecipitated (IP) with either (a) anti-C-terminal BACE1 (BACE1) antibody or (b) 2B3 antibody and then probed with 2B3 or BACE1 antibody. These results as presented are representative of three independent experiments with n = 3 for each condition. (c) Mouse brain homogenates prepared from TgsAPP-α mice and wild-type littermates (WT), and subjected to IP with BACE1 antibody for BACE1 followed by IB for sAPP-α using 2B3 antibody. The results as presented are representative of results obtained for 3 mice per group. (d) The conditioned media collected from cultured N2a/sAPP-α cells were subjected to IP with BACE1 antibody or isotype control IgG for BACE1 followed by IB with 6E10 antibody. As indicated below these blots, IB analyses demonstrate total protein levels of around 75 kDa BACE1, and 100 kDa sAPP-α in the different cell lysates, conditioned media or mouse brain homogenates. (e) sAPP-α strongly associates with BACE1 in N2a cell line stably transfected with human sAPP-α (N2a/sAPP-α cells). The cultured N2a/sAPP-α cells were fixed and imaged in independent channels using a confocal microscope equipped with Normarski optics. Merged images showed strong localization of both 6E10 antibody and BACE1 antibody positive N2a/sAPP-α cells (scale bar: 10 μm).