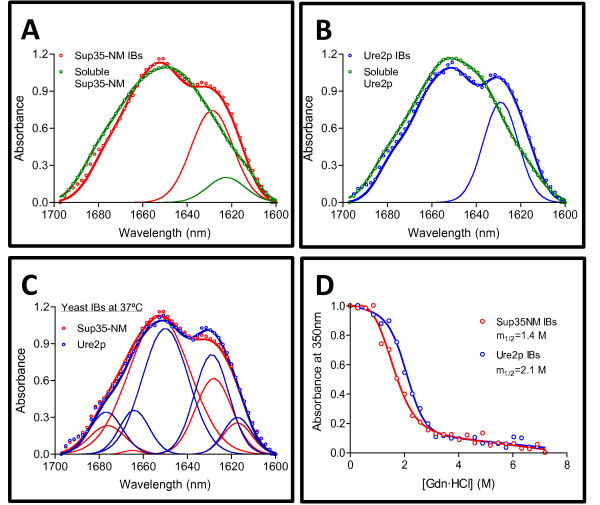
Figure 2.
Conformational properties of soluble and aggregated Sup35-NM and Ure2p proteins. Secondary structure of Sup35-NM (A) and Ure2p (B) yeast proteins in their soluble forms and inside the IBs formed at 37°C as determined FT-IR spectroscopy in the amide I region of the spectrum. Empty circles, solid thick lines and solid thin line show the absorbance spectra, the sum of individual spectral components and the inter-molecular β-sheet band, respectively; note that whereas Sup35-NM and Ure2p IBs display the typical inter-molecular β-sheet band at 1625–1630 cm-1, this signal is low or absent in soluble species. (C) Comparative analysis of the secondary structure of Sup35-NM and Ure2p IBs. Empty circles, solid thick lines and solid thin lines show the absorbance spectra, the sum of individual spectral components and the deconvolved component bands, respectively. (D) Stability of yeast prionogenic IBs in front of Gdn·HCl denaturation at equilibrium monitored by changes in turbidity at 350 nm.