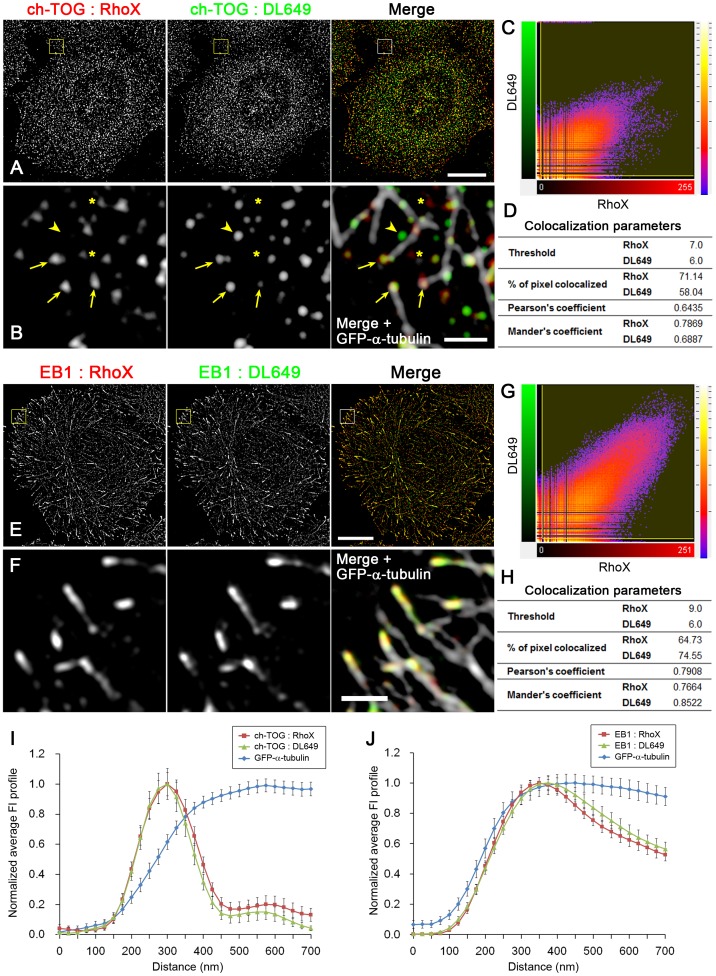
Figure 3. Evaluation of antibodies for EB1 and ch-TOG staining.
To test the specificities of the antibodies and the effects of fluorophores (emission wavelength), ch-TOG (A) and EB1 (E) were labelled with two different secondary antibodies conjugated with Rhodamine Red-X (RhoX) or DyLight 649 (DL649), and visualised by SIM. The RhoX and DL649 channels are merged in the right panels in red and green, respectively. In (B) and (F), boxed areas in (A) and (E), respectively, are enlarged and merged in the right panels together with GFP-α-tubulin signals (white). In (B), both RhoX and DL649 signals are detected in the same clusters localised at microtubule ends (arrows), although their signal intensities are not necessarily equal probably owing to competing binding to the same antigen, while clusters positive only for RhoX (asterisks) or DL649 (arrowheads) are also observed. The colocalisation of RhoX and DL649 signals was analysed and the scatter plots of the pixel intensities for the RhoX and DL649 channels and their colocalisation parameters are shown, respectively, in (C) and (D) for ch-TOG and in (G) and (H) for EB1. The values indicating the colocalisation of the RhoX and DL649 channels show that 30–40% of pixels were not colocalised when analysed at the individual pixel level. However, considering that the same cluster often appears to be a different size when labelled with two different antibodies as shown in (B), the colocalisation percentage may be underestimated. (I) Using the ch-TOG-double labelling images including (A), ch-TOG-positive microtubule ends not overlapping with other microtubules were selected and the FI profiles were averaged (n = 52, 5 cells in 3 images). The error bars are SEM. (J) Using the EB1-double labelling images including (E), EB1-positive microtubule ends not overlapping with other microtubules were selected and the FI intensities were averaged (n = 52, 2 cells in 2 images). The error bars are SEM.